2025-03-21 東京科学大学
<関連情報>
- https://www.isct.ac.jp/ja/news/sx6g54x3acgi
- https://www.isct.ac.jp/plugins/cms/component_download_file.php?type=2&pageId=&contentsId=1&contentsDataId=1079&prevId=&key=40fdc77c9820f2290cb8f78b57a85364.pdf
- https://translational-medicine.biomedcentral.com/articles/10.1186/s12967-025-06208-9
マイクロRNA-27aを導入した歯髄幹細胞は、in vitroでWnt/BMPシグナル伝達のDKK3およびSOSTDC1を標的として歯原性/骨原性分化を受け、in vivoで骨形成を促進する MicroRNA-27a transfected dental pulp stem cells undergo odonto/osteogenic differentiation via targeting DKK3 and SOSTDC1 in Wnt/BMP signaling in vitro and enhance bone formation in vivo
Ziniu Yu,Nobuyuki Kawashima,Keisuke Sunada-Nara,Shihan Wang,Peifeng Han,Thoai Quoc Kieu,Chunmei Ren,Sonoko Noda,Kento Tazawa & Takashi Okiji
Journal of Translational Medicine Published:16 February 2025
DOI:https://doi.org/10.1186/s12967-025-06208-9
Abstract
Background
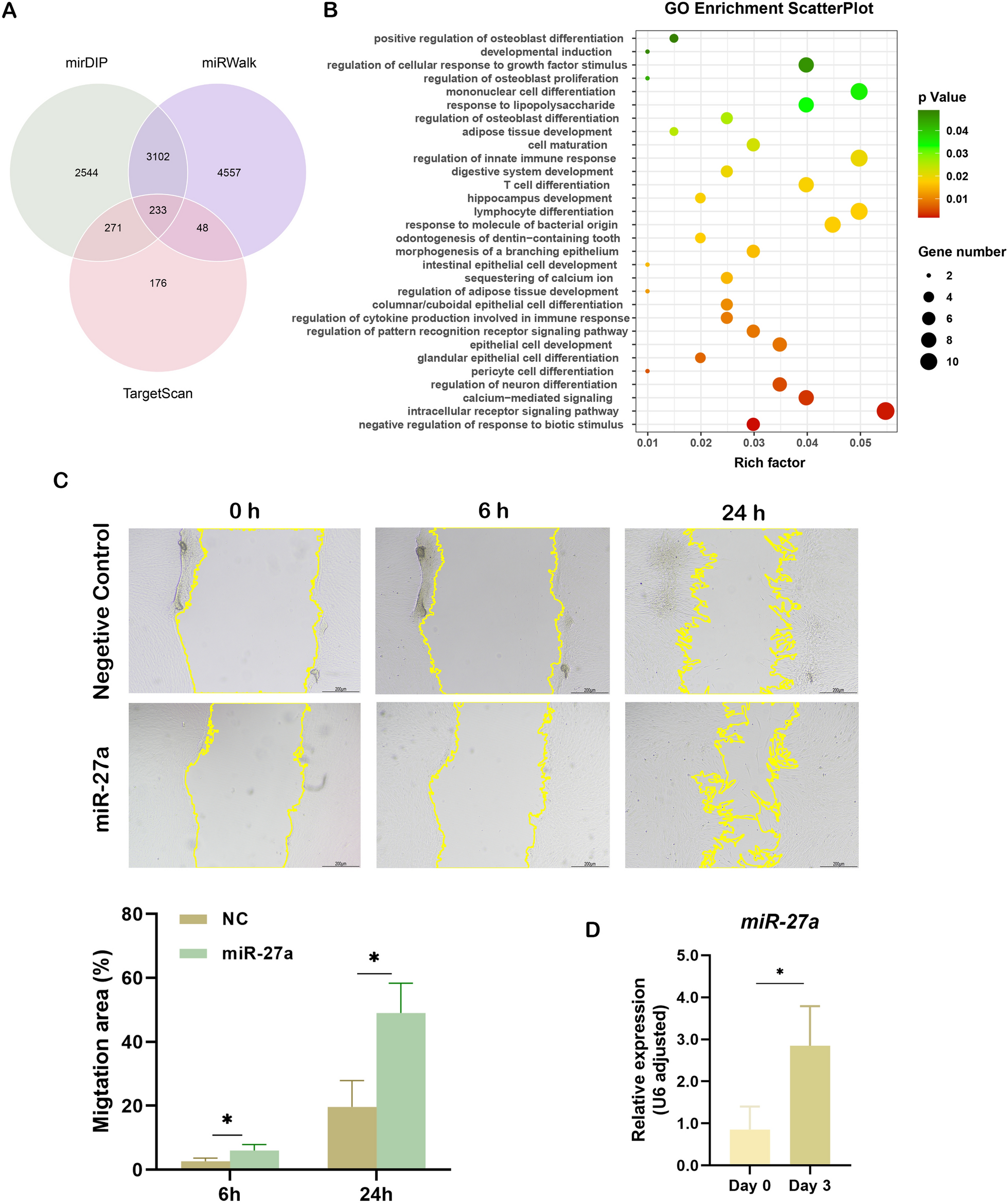
MicroRNAs (miRNAs) play a crucial role in cell differentiation through epigenetic regulation of gene expression. In human dental pulp cells, we have identified miRNA-27a being upregulated under inflammatory conditions. Here, we aimed to examine whether (i) overexpression of miRNA-27a in human dental pulp stem cells (hDPSCs) enhances their odonto/osteoblastic differentiation via Wnt and bone morphogenetic protein signaling; and (ii) hDPSCs overexpressing miRNA-27a promote new bone formation in vivo.
Methods
hDPSCs were cultured in osteogenic medium to promote differentiation. To examine the role of miRNA-27a, hDPSCs were transfected with either a miRNA-27a mimic to enhance or an inhibitor to suppress miRNA-27a expression. Odonto/osteoblastic differentiation was assessed by evaluating the expression of specific markers, Wnt and bone morphogenetic protein (BMP) signaling molecules, and mineralization capacity using RT-qPCR, western blotting, Alizarin Red S (ARS) staining, and alkaline phosphatase (ALP) activity. Potential miRNA-27a binding sites in the 3’UTRs of DKK3 and SOSTDC1 were identified via bioinformatics analysis and validated through the luciferase reporter assay. In vivo, miRNA-27a-overexpressing hDPSCs were seeded into collagen honeycomb scaffolds and implanted into mouse calvarial bone cavities to assess new bone formation.
Results
MiRNA-27a was highly upregulated in hDPSCs committed to odonto/osteoblastic differentiation. Overexpression of miRNA-27a led to increased expression of odonto/osteoblastic markers and enhanced mineralization capacity, while inhibition of miRNA-27a had the opposite effect. MiRNA-27a targeted DKK3, promoting β-catenin nuclear translocation and inhibiting SOSTDC1, which enhanced SMAD1/5 phosphorylation. Binding sites for miRNA-27a were identified in the 3’UTRs of DKK3 and SOSTDC1. In vivo, miRNA-27a-overexpressing hDPSCs promoted new bone formation in mouse calvaria bone cavities.
Conclusion
Transfection of miRNA-27a in hDPSCs enhanced their odonto/osteoblastic differentiation by targeting DKK3 and SOSTDC1, thereby promoting the Wnt and BMP signaling. Transplantation of miRNA-27a-overexpressing hDPSCs promoted new bone formation in vivo. These findings deepen our understanding of the effects of miRNA on Wnt and BMP pathways and suggest a potential clinical application for miRNA-27a in promoting hard tissue regeneration, offering a promising therapeutic target for dental and craniofacial tissue reconstruction.


