2025-03-21 中国科学院(CAS)
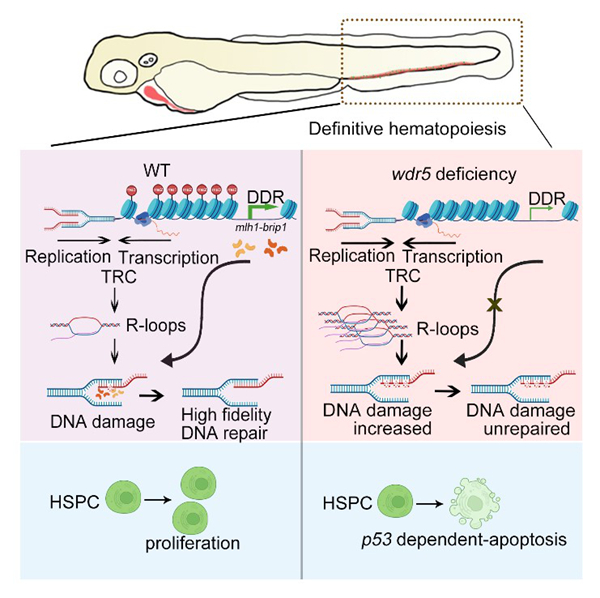
Schematic Diagram depicting the role of Wdr5-mediated H3K4 methylation in HSPC development (Image by Prof. LIU’s group)
<関連情報>
- https://english.cas.cn/newsroom/research_news/life/202503/t20250324_908557.shtml
- https://www.pnas.org/doi/10.1073/pnas.2420534122
Wdr5-mediated H3K4 methylation facilitates HSPC development via maintenance of genomic stability in zebrafish
Xiaohan Wang, Mengyao Liu, Yifan Zhang, +2 , and Feng Liu
Proceedings of the National Academy of Sciences Published:March 20, 2025
DOI:https://doi.org/10.1073/pnas.2420534122
Significance
Hematopoietic stem and progenitor cells (HSPCs) undergo rapid proliferation during embryonic development, establishing the foundation for lifelong hematopoiesis. Genomic stability is crucial for HSPC proliferation; however, the precise regulation of genomic stability to ensure normal development of HSPCs remains to be elucidated. In this study, we investigated the role of H3 lysine 4 (H3K4) methylation in developmental hematopoiesis and revealed its function in maintaining genomic stability within the proliferative HSPCs. We found that tryptophan-aspartic acid (WD) repeat protein 5 (Wdr5), a core scaffold subunit of H3K4 methyltransferase complex, is essential for reducing DNA damage via preventing the accumulation of R-loops. Furthermore, Wdr5-mediated H3K4 trimethylation is necessary for the activation of DNA damage response (DDR) signaling pathway, thereby facilitating genome protection in HSPC proliferation.
Abstract
During fetal stage, hematopoietic stem and progenitor cells (HSPCs) undergo rapid proliferation with a tight control of genomic stability. Although histone H3 lysine 4 (H3K4) methylation has been reported to stabilize the genome in proliferating cells, its specific role in HSPC development remains elusive. In this study, we demonstrated that tryptophan-aspartic acid (WD) repeat protein 5 (Wdr5)-mediated H3K4 methylation is crucial for maintaining genomic stability of proliferating HSPCs in zebrafish embryos. Loss of wdr5 led to a severe reduction of HSPC pool in the caudal hematopoietic tissue, accompanied with attenuated H3K4 methylation level and evident p53-dependent apoptosis in the HSPCs. Mechanistically, Wdr5-mediated H3K4 methylation maintains genomic stability by inhibiting the formation of abnormal R-loops in the HSPCs, whereas accumulation of R-loops exacerbates DNA damage. Moreover, the absence of H3K4 trimethylation leads to an inactivated DNA damage response (DDR) pathway, which is deleterious to DNA damage repair and genomic stability. Subsequently, we found that DDR-associated genes, mutL homolog 1 and breast and ovarian cancer interacting helicase 1, are important to ensure HSPC survival, likely by stabilizing their genome. In summary, these findings reveal that Wdr5-mediated H3K4 methylation is essential for HSPC development through tight control of R-loop accumulation and DDR-associated program to ensure genomic stability and survival of proliferating HSPCs.