2025-03-28 北海道大学,京都大学,大阪大学
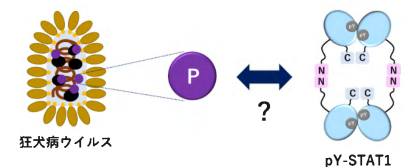
図 1. 本研究の概念図。
<関連情報>
- https://www.hokudai.ac.jp/news/2025/03/py-stat1stat.html
- https://www.hokudai.ac.jp/news/pdf/250328_pr3.pdf
- https://www.science.org/doi/10.1126/scisignal.ads2210
狂犬病ウイルスP蛋白質による、四量体チロシンリン酸化STAT1認識機構の構造学的解析 Structural analysis reveals how tetrameric tyrosine-phosphorylated STAT1 is targeted by the rabies virus P-protein
Aoi Sugiyama, Miku Minami, Kaito Ugajin, Satomi Inaba-Inoue, […], and Toyoyuki Ose
Science Signaling Published:18 Mar 2025
DOI:https://doi.org/10.1126/scisignal.ads2210
Editor’s summary
Because the STAT family of transcriptional regulators induces the expression of genes involved in antiviral signaling, they are often targeted by viruses to suppress immune responses. Activation of STATs by tyrosine phosphorylation induces their dimerization, and STAT dimers can assemble into tetramers with different transcriptional properties. Sugiyama et al. solved the cryo-EM structure of a tetrameric form of tyrosine-phosphorylated STAT1 in complex with DNA, showing how interactions between N-terminal domains of the protein supported the dimerization of STAT1 dimers. Biochemical analyses and modeling further indicated how the rabies virus P-protein specifically targets STAT1 tetramers, suggesting a potential mechanism of immune evasion. —John F. Foley
Abstract
Signal transducer and activator of transcription (STAT) family members mediate signaling in the Janus kinase (JAK)–STAT pathway and are activated by phosphorylation at a conserved tyrosine residue, resulting in dimerization through reciprocal interactions between the phosphotyrosine and a Src homology 2 (SH2) domain. Tyrosine-phosphorylated STAT (pY-STAT) then translocates to the nucleus to induce the expression of genes encoding antiviral proteins. Although the active and functional forms of STATs are conventionally considered to be dimers, STATs can undergo higher-order oligomerization, which is implicated in regulating transcriptional activity. We present the cryo–electron microscopy (cryo-EM) structure of the tetrameric form of intact pY-STAT1 in complex with DNA, which indicates that interactions between the amino-terminal domains (NTDs) of STAT1 induce oligomerization. The tetrameric structure revealed a compact conformation with a previously uncharacterized binding interface: Two DNA-bound dimers are twofold symmetrically aligned to transform into a tandem DNA-binding model without NTD dimer separation. Moreover, biochemical analyses indicated that the rabies virus P-protein selectively targeted tetrameric pY-STAT1. Combined with data showing which regions contribute to the interaction between pY-STAT1 and the P-protein, we constructed a binding model explaining how P recognizes the pY-STAT1 tetramer. These data provide insight into how pathogenic viruses target signaling pathways that mediate the host immune response.