2025-05-23 サセックス大学
<関連情報>
- https://www.sussex.ac.uk/research/full-news-list?id=68174
- https://molecular-cancer.biomedcentral.com/articles/10.1186/s12943-025-02346-2
LMTK3によるEVの生合成とカーゴソーティングの制御は、乳がんにおいて単球浸潤を減少させ、腫瘍化促進性マクロファージの分極を促進することにより腫瘍の成長を促進する LMTK3 regulation of EV biogenesis and cargo sorting promotes tumour growth by reducing monocyte infiltration and driving pro-tumourigenic macrophage polarisation in breast cancer
Mark Samuels,Christos Karakostas,Simoni Besta,Andrea Lauer Betrán,Katerina Tsilingiri,Charlotte Turner,Reza Shirazi Nia,Niloufar Poudine,Richard Goodyear,William Jones,Apostolos Klinakis & Georgios Giamas
Nature Cancer Published:23 May 2025
DOI:https://doi.org/10.1186/s12943-025-02346-2
Abstract
Background
Lemur Tail Kinase 3 (LMTK3) promotes cell proliferation, invasiveness and therapy resistance, and its expression correlates with poor survival in several different malignancies, including breast cancer. Crosstalk through extracellular vesicles (EVs) is an increasingly appreciated mechanism of cell communication within the tumour immune microenvironment, which contributes to different aspects of cancer progression and plays a pivotal role in shaping tumour fate.
Methods
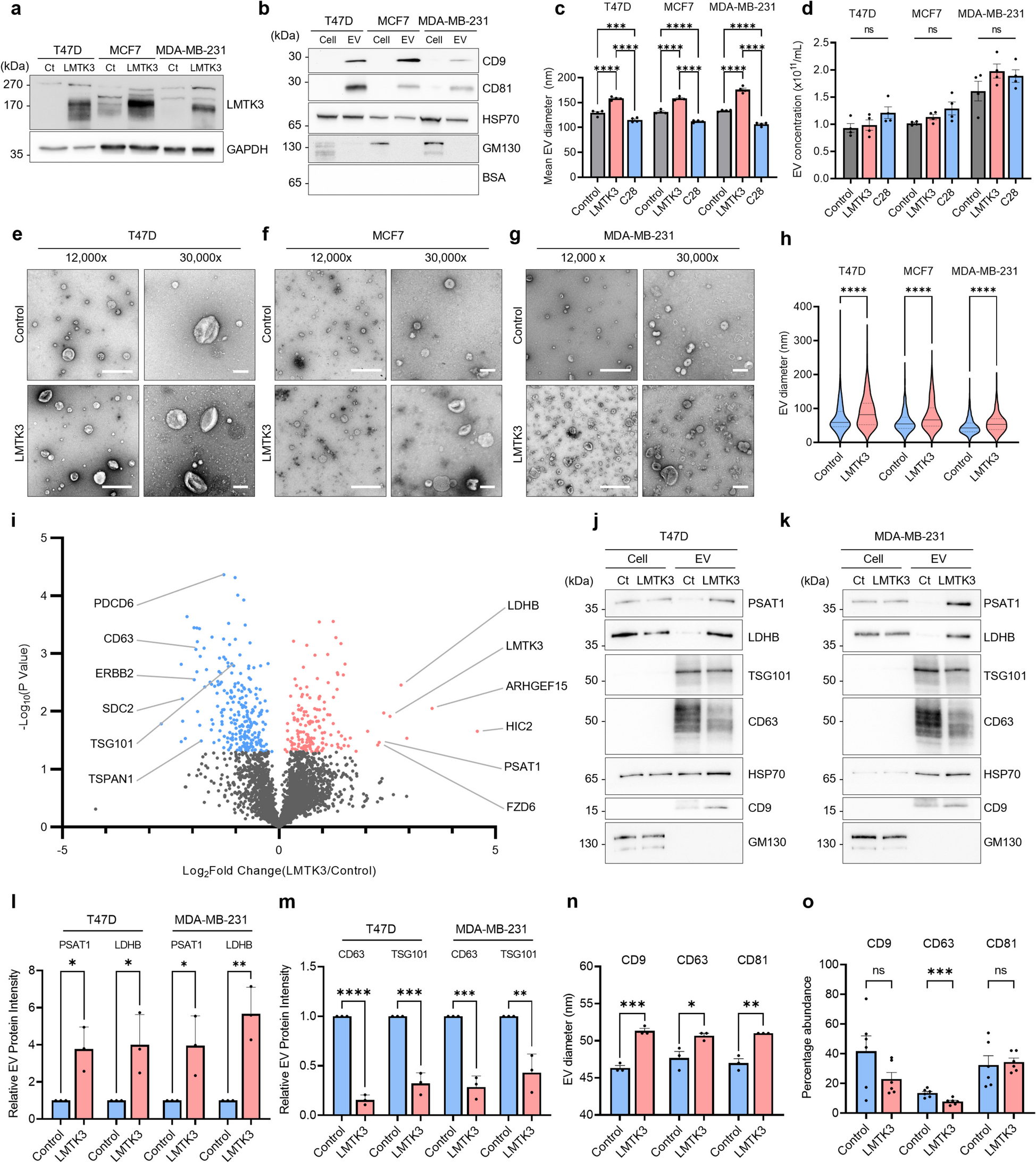
Nanoparticle tracking analysis and transmission electron microscopy were used to study the effects of LMTK3 on EV size, while single particle interferometry allowed us to examine LMTK3-dependent effects on the subpopulation distribution of EVs. Quantitative mass spectrometry was used to profile LMTK3-dependent proteomics changes in breast cancer-derived EVs. Bioinformatics analysis of clinical data along with in vitro and cell-based assays were implemented to explore the effects of LMTK3-dependent EV protein cargo on the tumour immune microenvironment. To elucidate the mechanism through which LMTK3 impacts endosomal trafficking and regulates EV biogenesis, we used a variety of approaches, including in vitro kinase assays, confocal and electron microscopy, as well as in vivo subcutaneous and orthotopic breast cancer mouse models.
Results
Here, we report that LMTK3 increases the average size of EVs, modulates immunoregulatory EV proteomic cargo and alters the subpopulation distribution of EVs released by breast cancer cells. Mechanistically, we provide evidence that LMTK3 phosphorylates Rab7, a key regulator of multivesicular body (MVB) trafficking, thereby reducing the fusion of MVBs with lysosomes and subsequent degradation of intralumenal vesicles, resulting in altered EV release. Moreover, LMTK3 causes increased packaging of phosphoserine aminotransferase 1 (PSAT1) in EVs, leading to a paracrine upregulation of phosphoglycerate dehydrogenase (PHGDH) in monocytes when these EVs are taken up. PSAT1 and PHGDH play key roles in the serine biosynthesis pathway, which is closely linked to cancer progression and regulation of monocyte behaviour. LMTK3 EV-induced elevated PHGDH expression in monocytes reduces their infiltration into breast cancer 3D spheroids and in vivo breast cancer mouse models. Furthermore, these infiltrating monocytes preferentially differentiate into pro-tumourigenic M2-like macrophages. Additional breast cancer mouse studies highlight the contribution of LMTK3-dependent EVs in the observed immunosuppressive macrophage phenotype. Finally, in vitro experiments show that pharmacological inhibition of LMTK3 reverses the pro-tumourigenic and immunomodulatory effects mediated by EVs derived from LMTK3 overexpressing cells.
Conclusion
Overall, this study advances our knowledge on the mechanisms of EV biogenesis and highlights a novel oncogenic role of LMTK3 in the breast TME, further supporting it as a target for cancer therapy.