2025-05-27 イェール大学
<関連情報>
- https://medicine.yale.edu/internal-medicine/news-article/phage-therapy-may-treat-drug-resistance-in-patients-with-cystic-fibrosis-study-finds/
- https://www.nature.com/articles/s41591-025-03678-8
嚢胞性線維症における多剤耐性緑膿菌治療のための個別化吸入バクテリオファージ療法 Personalized inhaled bacteriophage therapy for treatment of multidrug-resistant Pseudomonas aeruginosa in cystic fibrosis
Benjamin K. Chan,Gail L. Stanley,Kaitlyn E. Kortright,Albert C. Vill,Mrinalini Modak,Isabel M. Ott,Ying Sun,Silvia Würstle,Casey N. Grun,Barbara I. Kazmierczak,Govindarajan Rajagopalan,Zachary M. Harris,Clemente J. Britto,Jill Stewart,Jaideep S. Talwalkar,Casey R. Appell,Nauman Chaudary,Sugeet K. Jagpal,Raksha Jain,Adaobi Kanu,Bradley S. Quon,John M. Reynolds,Charlotte C. Teneback,Quynh-Anh Mai,… Jonathan L. Koff
Nature Medicine Published:29 April 2025
DOI:https://doi.org/10.1038/s41591-025-03678-8
Abstract
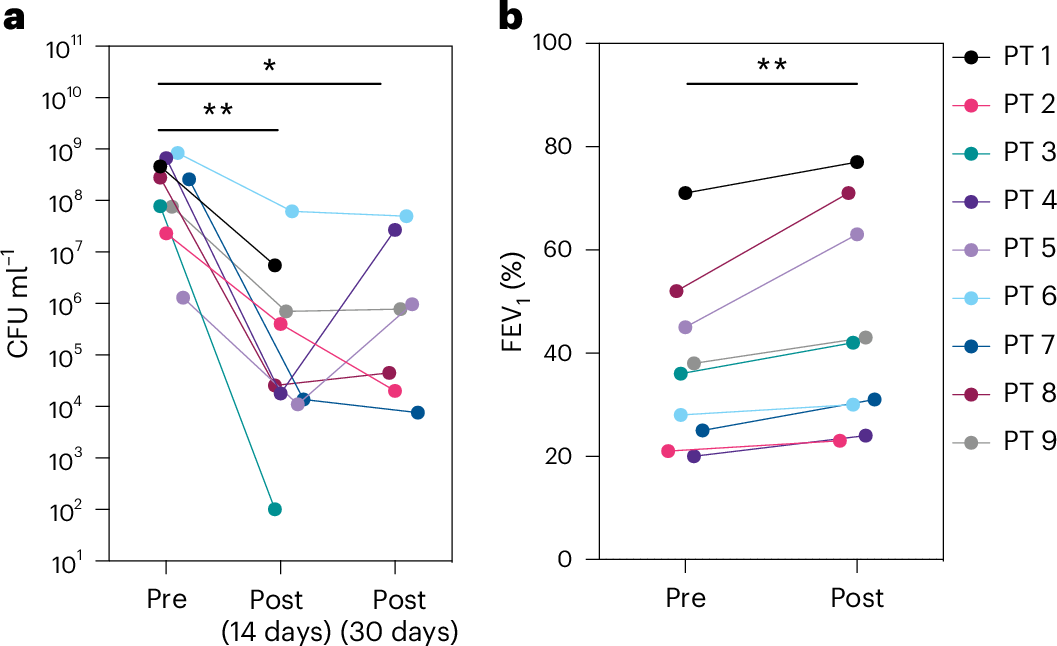
Bacteriophage (phage) therapy, which uses lytic viruses as antimicrobials, is a potential strategy to address the antimicrobial resistance crisis. Cystic fibrosis, a disease complicated by recurrent Pseudomonas aeruginosa pulmonary infections, is an example of the clinical impact of antimicrobial resistance. Here, using a personalized phage therapy strategy that selects phages for a predicted evolutionary trade-off, nine adults with cystic fibrosis (eight women and one man) of median age 32 (range 22–46) years were treated with phages on a compassionate basis because their clinical course was complicated by multidrug-resistant or pan-drug-resistant Pseudomonas that was refractory to prior courses of standard antibiotics. The individuals received a nebulized cocktail or single-phage therapy without adverse events. Five to 18 days after phage therapy, sputum Pseudomonas decreased by a median of 104 CFU ml-1, or a mean difference of 102 CFU ml-1 (P = 0.006, two-way analysis of variance with Dunnett’s multiple-comparisons test), without altering sputum microbiome, and an analysis of sputum Pseudomonas showed evidence of trade-offs that decreased antibiotic resistance or bacterial virulence. In addition, an improvement of 6% (median) and 8% (mean) predicted FEV1 was observed 21–35 days after phage therapy (P = 0.004, Wilcoxon signed-rank t-test), which may reflect the combined effects of decreased bacterial sputum density and phage-driven trade-offs. These results show that a personalized, nebulized phage therapy trade-off strategy may affect clinical and microbiologic endpoints, which must be evaluated in larger clinical trials.


