2025-06-27 東京大学
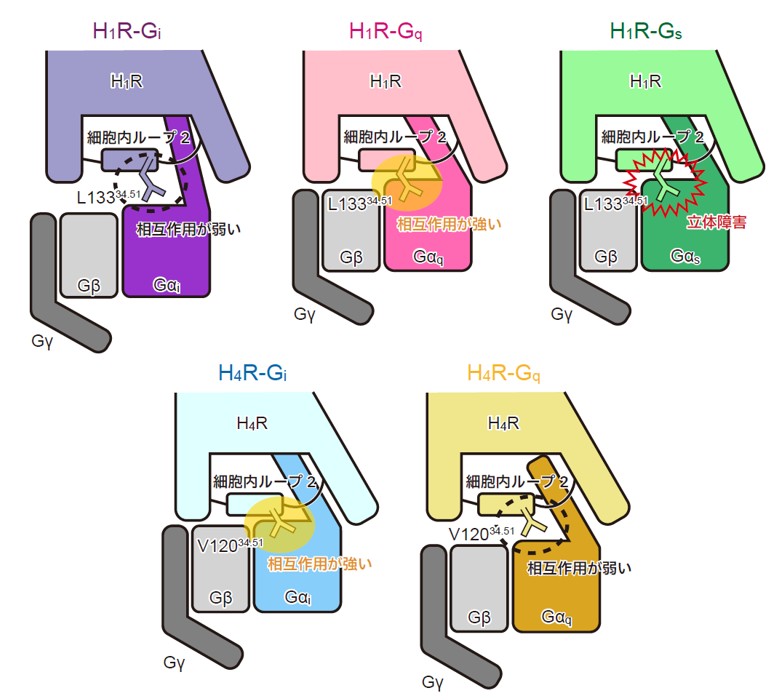
H1R/H4R と各Gタンパク質との相互作用の模式図
<関連情報>
ヒスタミン受容体におけるリガンド認識とGタンパク質選択性に関する構造的洞察 Structural insights into ligand recognition and G protein preferences across histamine receptors
Yuma Matsuzaki,Fumiya K. Sano,Hidetaka S. Oshima,Hiroaki Akasaka,Kazuhiro Kobayashi,Tatsuki Tanaka,Yuzuru Itoh,Wataru Shihoya,Yoshiaki Kise,Tsukasa Kusakizako,Asuka Inoue & Osamu Nureki
Communications Biology Published:27 June 2025
DOI:https://doi.org/10.1038/s42003-025-08363-7
Abstract
Histamine exerts critical physiological roles by activating four receptor subtypes, each exhibiting a specific G protein preference. Among these, the histamine H4 receptor (H4R) modulates chemotaxis and interferon production through Gi protein activation, suggesting its therapeutic potential. Despite its physiological significance, the mechanisms underlying H4R signalling and G protein preference across histamine receptors remain poorly understood. Here, we present the cryo-electron microscopy structure of the H4R-Gi complex, revealing unique mechanisms of histamine recognition and receptor activation. We further solved the structures of the histamine H1 receptor (H1R) bound to the non-canonical G proteins Gi and Gs. Through a combination of functional and computational analyses, we identified the intracellular loop 2 as a critical determinant of G protein preference in H1R and H4R. Collectively, our comprehensive study revealed the structural basis for distinct mechanisms of ligand recognition and receptor activation, offering a profound insight into G protein preference across receptor subtypes.