2025-07-30 中国科学院(CAS)
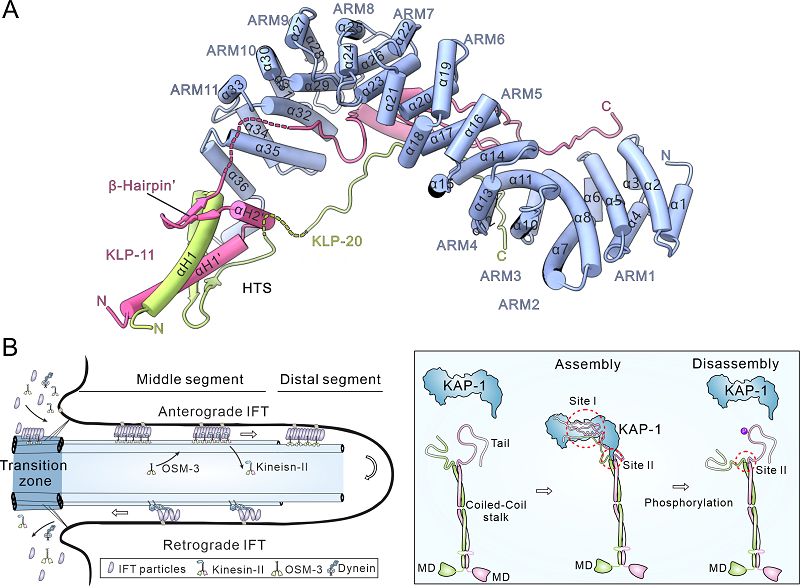 Overall structure of the tail region of the heterotrimeric kinesin-2 motor and schematic model of its role in regulating intraflagellar transport (IFT) (Image by FENG Wei’s group)
Overall structure of the tail region of the heterotrimeric kinesin-2 motor and schematic model of its role in regulating intraflagellar transport (IFT) (Image by FENG Wei’s group)
<関連情報>
- https://english.cas.cn/newsroom/research_news/life/202508/t20250801_1048955.shtml
- https://www.nature.com/articles/s41467-025-62152-8
相互認識メカニズムが、細胞内旗状体輸送のためのヘテロトリマー型キネシン-2の適切な組み立てを保証する A mutual co-recognition mechanism ensures the proper assembly of heterotrimeric kinesin-2 for intraflagellar transport
Jinqi Ren,Lingyan Zhao,Guanghan Chen,Guangshuo Ou & Wei Feng
Nature Communications Published:24 July 2025
DOI:https://doi.org/10.1038/s41467-025-62152-8
Abstract
Heterotrimeric kinesin-2, composed of two distinct kinesin motors and a kinesin-associated protein (KAP), is essential for intraflagellar transport and ciliogenesis. KAP specifically recognizes the hetero-paired motor tails for the holoenzyme assembly, but the underlying mechanism remains unclear. Here, we determine the structure of KAP-1 in complex with the hetero-paired tails from kinesin-2 motors KLP-20 and KLP-11. KAP-1 forms an elongated superhelical structure characterized by a central groove and a C-terminal helical (CTH)-hook. The two motor tails fold together and are co-recognized by the central groove of KAP-1. The adjacent hetero-pairing trigger sequences preceding the two tails form an intertwined heterodimer, which co-captures the CTH-hook of KAP-1 to complete the holoenzyme assembly. Mutations in the interfaces between KAP-1 and the two tails disrupt the heterotrimeric kinesin-2 complex and impair kinesin-2-mediated intraflagellar transport. Thus, KAP-1 and the hetero-paired motors are mutually co-recognized, ensuring the proper assembly of heterotrimeric kinesin-2 for cargo transport.