2025-12-19 大阪医科薬科大学,大阪大学,東北大学,龍谷大学,大阪公立大学,量子科学技術研究開発機構,神戸大学,理化学研究所,高輝度光科学研究センター,京都大学,兵庫県立大学
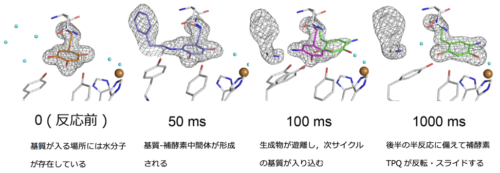
図1 触媒過程での補酵素TPQの構造変化
<関連情報>
- https://www.omp.ac.jp/public/press/pbp3vp0000001ia6.html
- https://www.omp.ac.jp/public/press/pbp3vp0000001ia6-att/pbp3vp0000001ign.pdf
- https://www.nature.com/articles/s41467-025-67230-5
ミックスアンドインジェクション連続結晶構造解析による銅アミン酸化酵素触媒中のドメイン運動のリアルタイム捕捉 Real-time capture of domain movements during copper amine oxidase catalysis by mix-and-inject serial crystallography
Takeshi Murakawa,Mamoru Suzuki,Kenji Fukui,Tetsuya Masuda,Eiichi Mizohata,Ikuko Miyahara,Ikuya Kurauchi,Taiki Murakami,Himawari Matsunaga,Yoshiki Montawa,Norie Nakajima,Toshinori Oozeki,Katsuki Sakai,Teikoku Son,Takehiro Higuchi,Tomoko Sunami,Tetsunari Kimura,Kensuke Tono,Tomoyuki Tanaka,Michihiro Sugahara,Toshi Arima,Luo Fangjia,Jungmin Kang,Rie Tanaka,… Toshihide Okajima
Nature Communications Published:18 December 2025
DOI:https://doi.org/10.1038/s41467-025-67230-5
Abstract
Protein dynamics play a crucial role in various physiological functions, including enzyme catalysis. To explore conformational changes during enzyme catalysis, we conduct mix-and-inject serial crystallography, an advanced technique to capture time-resolved protein structures in real time, using the microcrystals of bacterial copper amine oxidase containing a protein-derived quinone cofactor. Within 50 ms of mixing the microcrystals (<4 μm) with a preferred substrate (2-phenylethylamine) under anaerobic conditions (reductive half-reaction), we observe domain movements associated with substrate binding and formation of a metastable reaction intermediate, a product Schiff-base of the quinone cofactor. At 100–1000 ms after mixing, conformational transition from aminoresorcinol to the semiquinone radical forms of the reduced cofactor progresses gradually, likely depending on the replacement of the product aldehyde by the next-cycle amine substrate that triggers the cofactor conformational change. Overall, this study provides structural insight into enzyme catalysis accompanying the active-site conformational changes that are hardly scrutinized by studies in solution.


