2020-01-20 理化学研究所,MIT
理研-MIT神経回路遺伝学研究室の利根川進理研フェローらの研究チームは、恐怖の記憶が消去されることを喜びと感じるメカニズムの一端を明らかにしました。
本当に悪い経験が起こると予想し、そうでない場合、それは明らかに前向きな気持ちです。マウスでの恐怖絶滅訓練の新しい研究は、その理由を示唆する可能性があります:調査結果は、もはや恐怖を感じないことを学ぶための鍵となる脳細胞の正確な集団を特定するだけでなく、これらのニューロンが報酬の感情をエンコードするのに役立つものと同じであることも示しています。
MITのPicower Institute for Learning and Memoryの科学者が1月14日にNeuronで発表したこの研究は、恐怖の絶滅の記憶と報酬の感覚が、扁桃体後部(pBLA)の遺伝子Ppp1r1bを発現するニューロンによって同様に保存されることを具体的に示しています、嫌悪感ややりがいのある感情、または「バレンス」と記憶との関連付けを割り当てることが知られている地域。この研究は、大学院生のシャン・ギュー、元大学院生のジョシュア・キム、および理研-MITの生物学と神経科学の教授、利根川進、MITおよびハワードのピコワー学習および記憶研究所の神経回路遺伝学研究所によって実施されました。
詳細
January 15, 2020
RESEARCH FINDINGS
With these neurons, extinguishing fear is its own reward
When you expect a really bad experience to happen and then it doesn’t, it’s a distinctly positive feeling. A new study of fear extinction training in mice may suggest why: The findings not only identify the exact population of brain cells that are key for learning not to feel afraid anymore, but also show these neurons are the same ones that help encode feelings of reward.
The study, published Jan. 14 in Neuron by scientists at MIT’s Picower Institute for Learning and Memory, specifically shows that fear extinction memories and feelings of reward alike are stored by neurons that express the gene Ppp1r1b in the posterior of the basolateral amygdala (pBLA), a region known to assign associations of aversive or rewarding feelings, or “valence,” with memories. The study was conducted by Xiangyu Zhang, a graduate student, Joshua Kim, a former graduate student, and Susumu Tonegawa, Professor of Biology and Neuroscience at RIKEN-MIT Laboratory of Neural Circuit Genetics at the Picower Institute for Learning and Memory at MIT and Howard Hughes Medical Institute.
“We constantly live at the balance of positive and negative emotion,” Tonegawa said. “We need to have very strong memories of dangerous circumstances in order to avoid similar circumstances to recur. But if we are constantly feeling threatened we can become depressed. You need a way to bring your emotional state back to something more positive.”
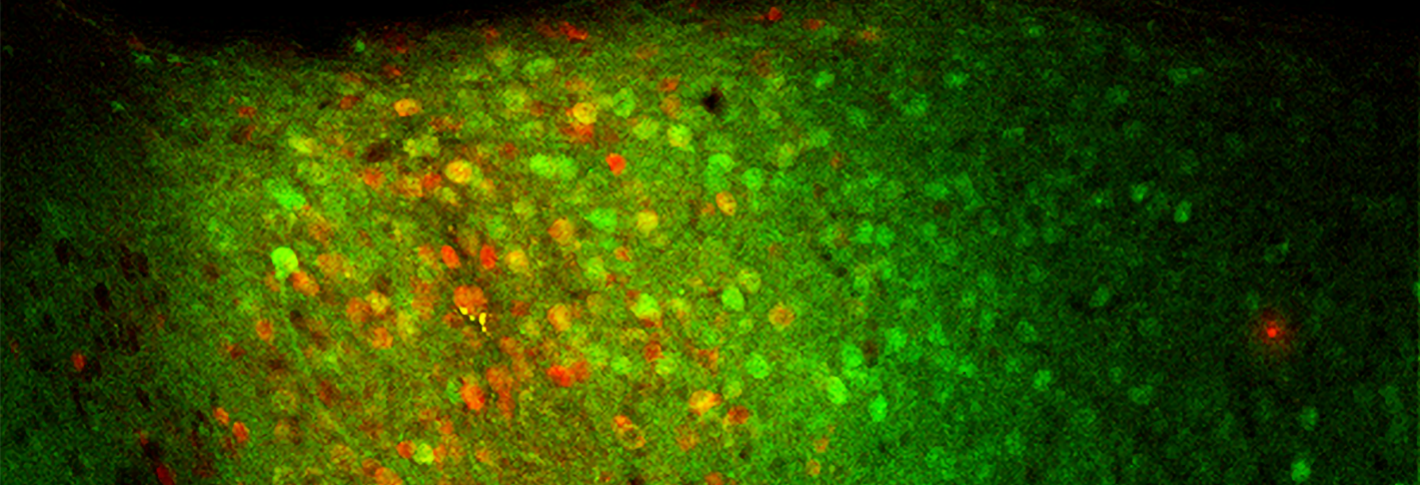
Above: In the basolateral amygdala of a mouse, Ppp1r1b-expressing cells are stained green, while the cells in a fear extinction memory engram appear red.
Overriding fear with reward
In a prior study, Kim showed that Ppp1r1b-expressing neurons encode rewarding valence and compete with distinct Rspo2-expressing neurons in the BLA that encode negative valence. In the new study, Zhang, Kim and Tonegawa set out to determine whether this competitive balance also underlies fear and its extinction.
In fear extinction, an original fearful memory is thought to be essentially overwritten by a new memory that is not fearful. In the study, for instance, mice were exposed to little shocks in a chamber, making them freeze due to the formation of fearful memory. But the next day, when the mice were returned to the same chamber for a longer period of time without any further little shocks, freezing gradually dissipated and hence this treatment is called fear extinction training. The fundamental question then is whether the fearful memory is lost or just suppressed by the formation of a new memory during the fear extinction training.
While the mice underwent fear extinction training the scientists watched the activity of the different neural populations in the BLA. They saw that Ppp1r1b cells were more active and Rspo2 cells were less active in mice that experienced fear extinction. They also saw that while Rspo2 cells were mostly activated by the shocks and were inhibited during fear extinction, Ppp1r1b cells were mostly active during extinction memory training and retrieval, but were inhibited during the shocks.
These and other experiments suggested to the authors that the hypothetical fear extinction memory may be formed in the Ppp1r1b neuronal population and the team went on to demonstrate this vigorously. For this, they employed the technique previously pioneered in their lab for the identification and manipulation of the neuronal population that holds specific memory information, memory “engram” cells. Zhang labeled Ppp1r1b neurons that were activated during retrieval of fear extinction memory with the light-sensitive protein channelrhodopsin. When these neurons were activated by blue laser light during a second round of fear extinction training it enhanced and accelerated the extinction. Moreover, when the engram cells were inhibited by another optogenetic technique, fear extinction was impaired because the Ppp1r1b engram neurons could no longer suppress the Rspo2 fear neurons. That allowed the fear memory to regain primacy.
These data met the fundamental criteria for the existence of engram cells for fear extinction memory within the pBLA Ppp1r1b cell population: activation and reactivation by recall and enduring and off-line maintenance of the acquired extinction memory.
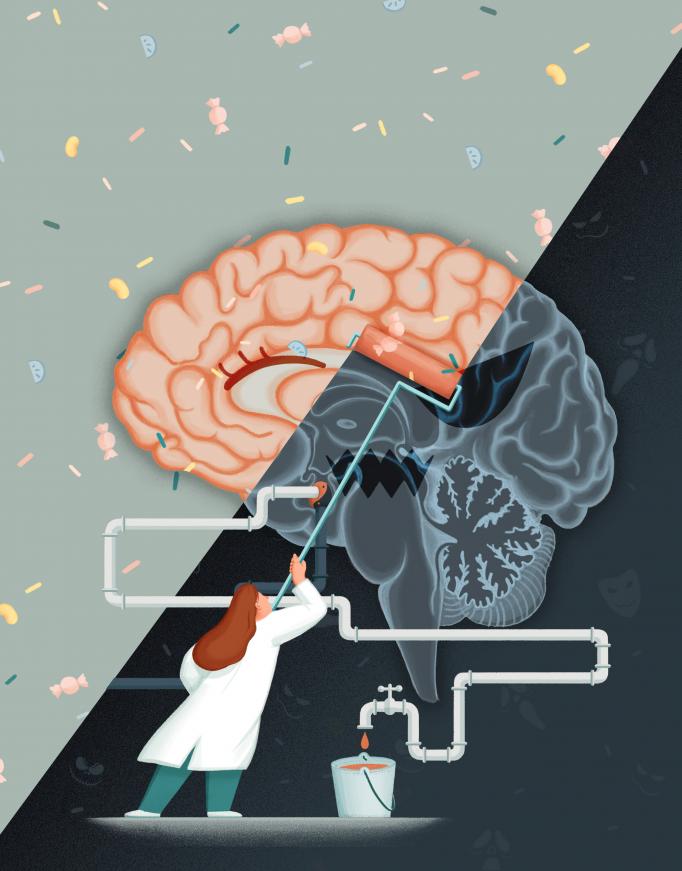
A central finding of the study is that a fear extinction memory stored in an engram of reward cells in the basolateral amygdala can suppress a more fearful memory stored by Rspo2 cells elsewhere in the BLA. Illustration by Yi Zheng.
Because Kim had previously shown Ppp1r1b neurons are activated by rewards and drive appetitive behavior and memory, the team sequentially tracked Ppp1r1b cell activity in mice that eagerly received water reward followed by food reward followed by fear extinction training and fear extinction memory retrieval. The overlap of Ppp1r1b neurons activated by fear extinction vs. water reward was as high as the overlap of neurons activated by water vs. food reward. And finally, artificial optogenetic activation of Ppp1r1b extinction memory engram cells was as effective as optogenetic activation of Ppp1r1b water reward-activated neurons in driving appetitive behaviors. Reciprocally, artificial optogenetic activation of water-responding Ppp1r1b neurons enhanced fear extinction training as efficiently as optogenetic activation of fear extinction memory engram cells. These results demonstrate that fear extinction is equivalent to bona fide rewards and therefore provide the neuroscientific basis for the widely held experience in daily life: omission of expected punishment is a reward.
What next?
By establishing this intimate connection between fear extinction and reward and by identifying a genetically defined neuronal population (Ppp1r1b) that plays a crucial role in fear extinction this study provides potential therapeutic targets for treating fear disorders like PTSD and anxiety, Zhang said.
From the basic scientific point of view, Tonegawa said, how fear extinction training specifically activates Ppp1r1b neurons would be an important question to address. More imaginatively, results showing how Ppp1r1b neurons override Rspo2 neurons in fear extinction raises an intriguing question about whether a reciprocal dynamic might also occur in the brain and behavior. Investigating “joy extinction” via these mechanisms might be an interesting research topic.
The research was supported by the RIKEN Brain Science Institute, the Howard Hughes Medical Institute and the JPB Foundation funded the research.
報道担当
理化学研究所 広報室 報道担当