2023-04-04 カリフォルニア大学リバーサイド校(UCR)
この研究は、このような生殖障害の理由を理解し、治療法を見つけることに役立つと考えられています。また、この研究によってリスクのある女性に子供を持つタイミングや健康管理のアドバイスをすることもできるようになるかもしれません。
<関連情報>
- https://news.ucr.edu/articles/2023/04/04/how-autism-gene-contributes-infertility
- https://www.frontiersin.org/articles/10.3389/fendo.2023.1129534/full
フラジャイルXメッセンジャーリボ核タンパク質(Fmr1)遺伝子変異による生殖機能障害の特徴であるGnRHニューロンおよび卵巣神経支配の異常
Altered GnRH neuron and ovarian innervation characterize reproductive dysfunction linked to the Fragile X messenger ribonucleoprotein (Fmr1) gene mutation
Pedro A. Villa, Nancy M. Lainez, Carrie R. Jonak, Sarah C. Berlin, Iryna M. Ethell and Djurdjica Coss
Frontiers in Endocrinology Published:22 February 2023
DOI:https://doi.org/10.3389/fendo.2023.1129534
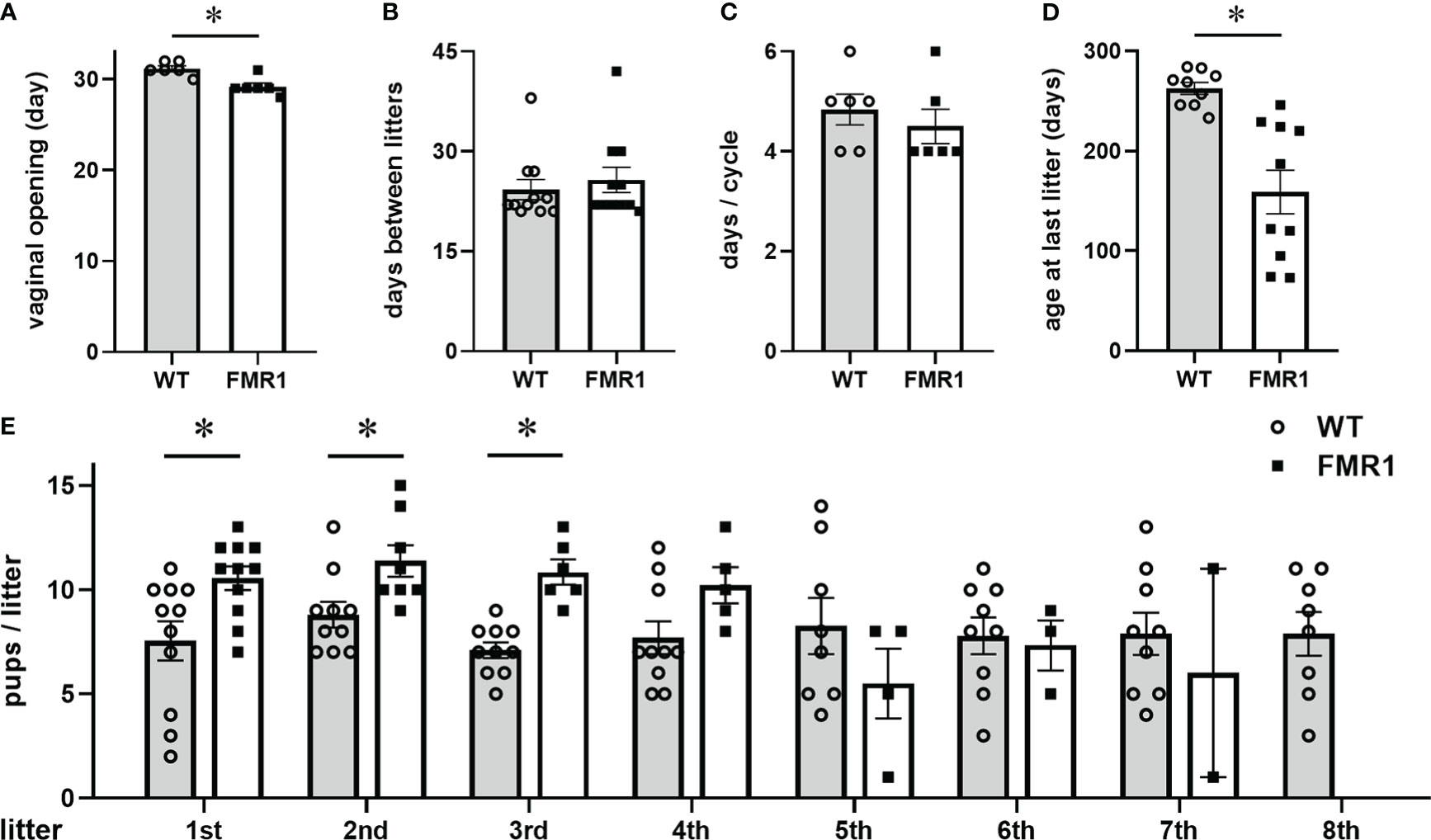
Introduction: Mutations in the Fragile X Messenger Ribonucleoprotein 1 (FMR1) gene cause Fragile X Syndrome, the most common monogenic cause of intellectual disability. Mutations of FMR1 are also associated with reproductive disorders, such as early cessation of reproductive function in females. While progress has been made in understanding the mechanisms of mental impairment, the causes of reproductive disorders are not clear. FMR1-associated reproductive disorders were studied exclusively from the endocrine perspective, while the FMR1 role in neurons that control reproduction was not addressed.
Results: Here, we demonstrate that similar to women with FMR1 mutations, female Fmr1 null mice stop reproducing early. However, young null females display larger litters, more corpora lutea in the ovaries, increased inhibin, progesterone, testosterone, and gonadotropin hormones in the circulation. Ovariectomy reveals both hypothalamic and ovarian contribution to elevated gonadotropins. Altered mRNA and protein levels of several synaptic molecules in the hypothalamus are identified, indicating reasons for hypothalamic dysregulation. Increased vascularization of corpora lutea, higher sympathetic innervation of growing follicles in the ovaries of Fmr1 nulls, and higher numbers of synaptic GABAA receptors in GnRH neurons, which are excitatory for GnRH neurons, contribute to increased FSH and LH, respectively. Unmodified and ovariectomized Fmr1 nulls have increased LH pulse frequency, suggesting that Fmr1 nulls exhibit hyperactive GnRH neurons, regardless of the ovarian feedback.
Conclusion: These results reveal Fmr1 function in the regulation of GnRH neuron secretion, and point to the role of GnRH neurons, in addition to the ovarian innervation, in the etiology of Fmr1-mediated reproductive disorders.
