核膜の孔に張り巡らされたダイナミックなネットワークが、危険な侵入者をブロックする Dynamic network in the pores of the nuclear envelope blocks dangerous invaders
2023-04-27 マックス・プランク研究所
ドイツのマインツヨハネス・グーテンベルク大学の合成バイオ物理学研究グループとフランクフルトのマックスプランクバイオ物理学研究所の理論バイオ物理学部門からなるチームは、核孔の構造と機能についての理解における重要な穴を埋めました。
彼らの研究により、核孔の中央にある本来無秩序なタンパク質が、スパゲッティのような可動的なバリアを形成することがわかりました。このバリアは、重要な細胞内因子を通すことができますが、ウイルスやその他の病原体をブロックすることができます。これにより、核孔は分子的な用心棒のような存在と考えることができます。
<関連情報>
- https://www.mpg.de/20233765/0427-biop-how-wiggly-spaghetti-proteins-guard-the-genome-153215-x?c=2249
- https://www.nature.com/articles/s41586-023-05990-0
無秩序な核輸送装置のその場での可視化 Visualizing the disordered nuclear transport machinery in situ
Miao Yu,Maziar Heidari,Sofya Mikhaleva,Piau Siong Tan,Sara Mingu,Hao Ruan,Christopher D. Reinkemeier,Agnieszka Obarska-Kosinska,Marc Siggel,Martin Beck,Gerhard Hummer & Edward A. Lemke
Nature Published:26 April 2023
DOI:https://doi.org/10.1038/s41586-023-05990-0
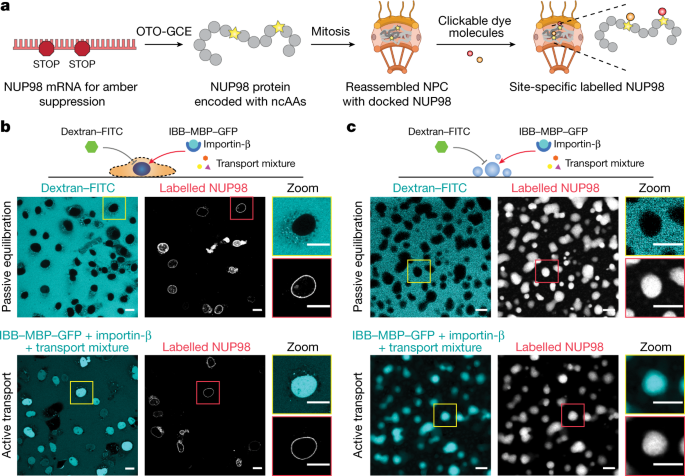
Abstract
The approximately 120 MDa mammalian nuclear pore complex (NPC) acts as a gatekeeper for the transport between the nucleus and cytosol1. The central channel of the NPC is filled with hundreds of intrinsically disordered proteins (IDPs) called FG-nucleoporins (FG-NUPs)2,3. Although the structure of the NPC scaffold has been resolved in remarkable detail, the actual transport machinery built up by FG-NUPs—about 50 MDa—is depicted as an approximately 60-nm hole in even highly resolved tomograms and/or structures computed with artificial intelligence4,5,6,7,8,9,10,11. Here we directly probed conformations of the vital FG-NUP98 inside NPCs in live cells and in permeabilized cells with an intact transport machinery by using a synthetic biology-enabled site-specific small-molecule labelling approach paired with highly time-resolved fluorescence microscopy. Single permeabilized cell measurements of the distance distribution of FG-NUP98 segments combined with coarse-grained molecular simulations of the NPC allowed us to map the uncharted molecular environment inside the nanosized transport channel. We determined that the channel provides—in the terminology of the Flory polymer theory12—a ‘good solvent’ environment. This enables the FG domain to adopt expanded conformations and thus control transport between the nucleus and cytoplasm. With more than 30% of the proteome being formed from IDPs, our study opens a window into resolving disorder–function relationships of IDPs in situ, which are important in various processes, such as cellular signalling, phase separation, ageing and viral entry.


