2023-10-25 ワシントン大学セントルイス校
◆この研究は、凝縮体が一種の有構造体であることを示し、正常状態と疾患状態を区別する基本原則を提供するもので、将来的には合成凝縮体の開発にも貢献する可能性があります。凝縮体は、細胞のストレスに応じて形成および解消する構造体であり、不適切な凝縮体の形成と解消は、ALSなどの人間の疾患と関連しています。
◆この研究により、凝縮体の組織化が明らかになり、疾患の発展を理解し、将来的には合成凝縮体の開発に役立つかもしれません。
<関連情報>
- https://engineering.wustl.edu/news/2023/Mapping-the-cells-membrane-less-compartments.html
- https://www.nature.com/articles/s41467-023-41274-x#citeas
タンパク質混合物の相分離はホモ型相互作用とヘテロ型相互作用の相互作用によって駆動される Phase separation of protein mixtures is driven by the interplay of homotypic and heterotypic interactions
Mina Farag,Wade M. Borcherds,Anne Bremer,Tanja Mittag & Rohit V. Pappu
Nature Communications Published:08 September 2023
DOI:https://doi.org/10.1038/s41467-023-41274-x
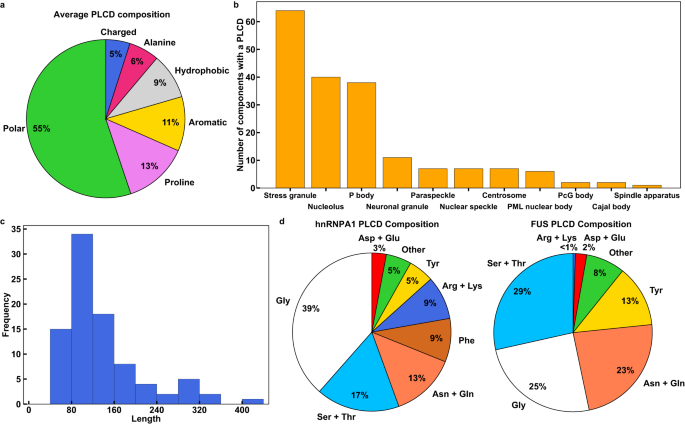
Abstract
Prion-like low-complexity domains (PLCDs) are involved in the formation and regulation of distinct biomolecular condensates that form via phase separation coupled to percolation. Intracellular condensates often encompass numerous distinct proteins with PLCDs. Here, we combine simulations and experiments to study mixtures of PLCDs from two RNA-binding proteins, hnRNPA1 and FUS. Using simulations and experiments, we find that 1:1 mixtures of A1-LCD and FUS-LCD undergo phase separation more readily than either of the PLCDs on their own due to complementary electrostatic interactions. Tie line analysis reveals that stoichiometric ratios of different components and their sequence-encoded interactions contribute jointly to the driving forces for condensate formation. Simulations also show that the spatial organization of PLCDs within condensates is governed by relative strengths of homotypic versus heterotypic interactions. We uncover rules for how interaction strengths and sequence lengths modulate conformational preferences of molecules at interfaces of condensates formed by mixtures of proteins.