2024-06-07 インペリアル・カレッジ・ロンドン(ICL)
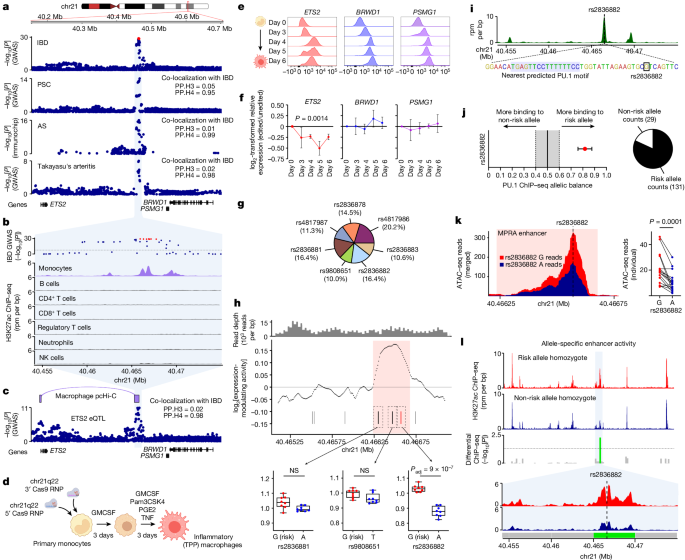
◆IBDは複雑な自己免疫疾患であり、現在英国では約50万人が罹患しています。既存の治療法が全ての患者に効果があるわけではなく、新薬開発の試みはしばしば失敗しています。研究者たちは非コードDNA領域を調査し、免疫細胞であるマクロファージで活性化される重要なエンハンサー領域を特定しました。この領域はETS2遺伝子の活動を増強し、IBDリスクを高めます。
◆MEK阻害剤はマクロファージの炎症を抑え、患者の腸サンプルでも効果が確認されましたが、副作用のため、今後はMEK阻害剤をマクロファージに直接届ける方法を模索しています。この研究はIBDの新しい治療法開発に向けた重要な一歩です。
<関連情報>
- https://www.imperial.ac.uk/news/253914/major-cause-inflammatory-bowel-disease-discovered/
- https://www.nature.com/articles/s41586-024-07501-1
ETS2を介してマクロファージの炎症を誘導する疾患関連遺伝子砂漠 A disease-associated gene desert directs macrophage inflammation through ETS2
C. T. Stankey,C. Bourges,L. M. Haag,T. Turner-Stokes,A. P. Piedade,C. Palmer-Jones,I. Papa,M. Silva dos Santos,Q. Zhang,A. J. Cameron,A. Legrini,T. Zhang,C. S. Wood,F. N. New,L. O. Randzavola,L. Speidel,A. C. Brown,A. Hall,F. Saffioti,E. C. Parkes,W. Edwards,H. Direskeneli,P. C. Grayson,L. Jiang,… J. C. Lee
Nature Published:05 June 2024
DOI:https://doi.org/10.1038/s41586-024-07501-1
Abstract
Increasing rates of autoimmune and inflammatory disease present a burgeoning threat to human health1. This is compounded by the limited efficacy of available treatments1 and high failure rates during drug development2, highlighting an urgent need to better understand disease mechanisms. Here we show how functional genomics could address this challenge. By investigating an intergenic haplotype on chr21q22—which has been independently linked to inflammatory bowel disease, ankylosing spondylitis, primary sclerosing cholangitis and Takayasu’s arteritis3,4,5,6—we identify that the causal gene, ETS2, is a central regulator of human inflammatory macrophages and delineate the shared disease mechanism that amplifies ETS2 expression. Genes regulated by ETS2 were prominently expressed in diseased tissues and more enriched for inflammatory bowel disease GWAS hits than most previously described pathways. Overexpressing ETS2 in resting macrophages reproduced the inflammatory state observed in chr21q22-associated diseases, with upregulation of multiple drug targets, including TNF and IL-23. Using a database of cellular signatures7, we identified drugs that might modulate this pathway and validated the potent anti-inflammatory activity of one class of small molecules in vitro and ex vivo. Together, this illustrates the power of functional genomics, applied directly in primary human cells, to identify immune-mediated disease mechanisms and potential therapeutic opportunities.