2024-10-23 ペンシルベニア州立大学(PennState)
Credit: Courtsey of Dokholyan lab / Penn State. Creative Commons
<関連情報>
- https://www.psu.edu/news/research/story/re-engineered-blue-light-activated-immune-cells-penetrate-and-kill-solid-tumors
- https://www.pnas.org/doi/10.1073/pnas.2405717121
光遺伝学的に操作されたセプチン-7が腫瘍スフェロイドへの免疫細胞の浸潤を促進する Optogenetically engineered Septin-7 enhances immune cell infiltration of tumor spheroids
Jiaxing Chen, Brianna Hnath, Congzhou M. Sha, +2, and Nikolay V. Dokholyan
Proceedings of the National Academy of Sciences Published:October 23, 2024
DOI:https://doi.org/10.1073/pnas.2405717121
Significance
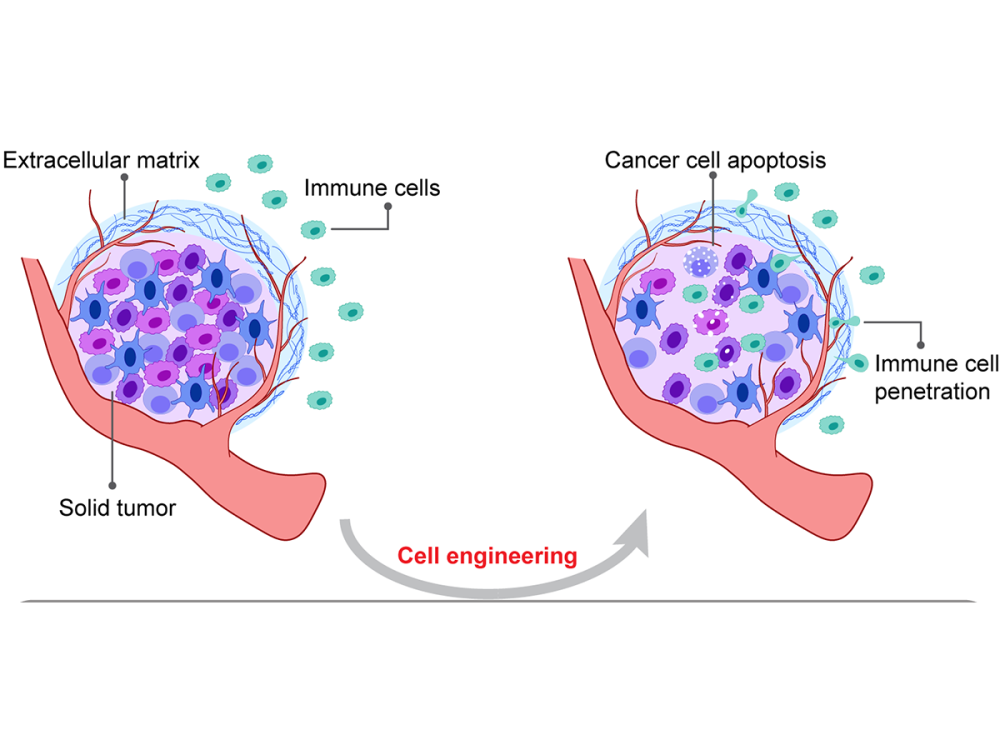
Cancer is a major public health concern and the second-most common cause of death worldwide. Traditional approaches for cancer treatment have been frustrating as they only extend patients’ lifetime along with sacrificing patients’ life quality. During the last decade, chimeric antigen receptor (CAR)-T therapy has emerged and shown great success in certain hematological malignancies including lymphoblastic leukemia and B cell lymphoma. However, the efficacy of using cellular immunotherapy to treat solid tumors is limited due to the poor tumor infiltration of immune cells. Here, we enhance immune cell penetration into tumor spheroids by manipulating septin-7 function in live cells, which might improve cell-based immunotherapies against solid tumors.
Abstract
Chimeric antigen receptor T cell therapies have achieved great success in eradicating some liquid tumors, whereas the preclinical results in treating solid tumors have proven less decisive. One of the principal challenges in solid tumor treatment is the physical barrier composed of a dense extracellular matrix, which prevents immune cells from penetrating the tissue to attack intratumoral cancer cells. Here, we improve immune cell infiltration into solid tumors by manipulating septin-7 functions in cells. Using protein allosteric design, we reprogram the three-dimensional structure of septin-7 and insert a blue light-responsive light-oxygen-voltage-sensing domain 2 (LOV2), creating a light-controllable septin-7-LOV2 hybrid protein. Blue light inhibits septin-7 function in live cells, inducing extended cell protrusions and cell polarization, enhancing cell transmigration efficiency through confining spaces. We genetically edited human natural killer cell line (NK92) and mouse primary CD8+ T-cells expressing the engineered protein, and we demonstrated improved penetration and cytotoxicity against various tumor spheroid models. Our proposed strategy to enhance immune cell infiltration is compatible with other methodologies and therefore, could be used in combination to further improve cell-based immunotherapies against solid tumors.