2025-07-19 東京大学
天然物骨格のリデザイン戦略による抗腫瘍性マクロ環状中分子群の創製
<関連情報>
- https://www.s.u-tokyo.ac.jp/ja/press/10859/
- https://www.cell.com/chem/abstract/S2451-9294(25)00255-4
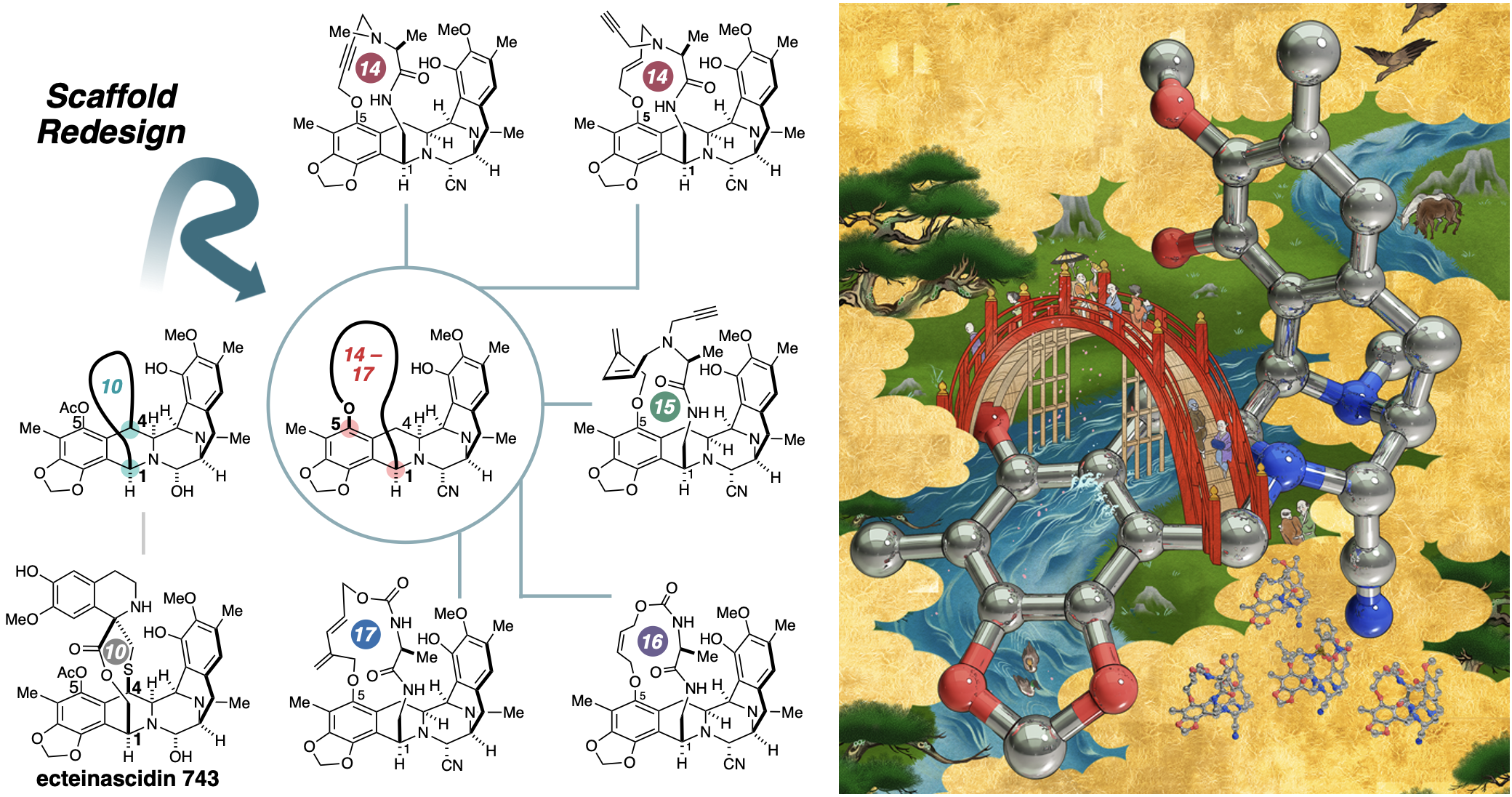
エクテナシジンの戦略的骨格再設計:抗癌性マクロサイクルを生成するためのアプローチ Strategic scaffold redesign of ecteinascidins: An approach for generating anticancer macrocycles
Ryo Tanifuji ∙ Erina Hosono ∙ Hisae Kamakura ∙ … ∙ Shingo Dan ∙ Hiroyuki Seimiya ∙ Hiroki Oguri
Chem Published:July 18, 2025
DOI:https://doi.org/10.1016/j.chempr.2025.102664
The bigger picture
Natural products have long served as a cornerstone of drug discovery, offering unparalleled complexity and bioactivity. However, their development as therapeutics is often constrained by synthetic challenges and intricate molecular architectures. Our study presents a transformative approach to streamline the molecular redesign of ecteinascidin 743 (ET-743), an FDA-approved anticancer drug. This strategy facilitates the efficient design and synthesis of novel macrocyclic derivatives with tunable pharmacological profiles, significantly reducing synthetic complexity while preserving the essential bioactivity of the drug. The synthetic variants of ecteinascidins we have developed are capable of reversibly alkylating DNA, thereby modulating interactions with both DNA and nuclear proteins. This broadens the horizons of natural product-inspired drug discovery. Ultimately, our approach has the potential to transform natural products into advanced therapies to fight fatal cancers, making these treatments more accessible.
Highlights
- Scaffold redesign enables divergent synthesis of 14- to 17-membered macrocycles
- C1–C5 bridging expands 3D chemical space beyond the C1–C4 ecteinascidin scaffold
- Late-stage diversification retains DNA alkylation and modulates anticancer activity
- Streamlined route reduces steps from 21 to 6–10, cutting synthetic burden by >50%
Summary
Strategies for rational design and scaffold diversification of therapeutically valuable yet synthetically challenging natural products remain elusive, often overshadowed by structural simplification approaches. Herein, we report the molecular redesign of an antitumor drug, ecteinascidin 743, which achieves three pivotal objectives: (1) strategic shift of the bridgehead position from C4 to C5, (2) systematic customization of macrocycle size, and (3) incorporation of functional groups for further modification. Our approach generates diverse 14- to 17-membered macrocyclic frameworks bridged at C5, expanding the accessible chemical space beyond that of conventional C1- to C4-bridged scaffolds, while preserving the core structure essential for covalent DNA interactions. These novel macrocycles induce DNA double-strand breaks and exhibit sub-nanomolar anticancer efficacy comparable to ecteinascidins. This method shortens the conventional 21-step semi-synthetic protocol into a streamlined 6- to 10-step process, cutting the synthetic burden by over 50%.