2025-08-20 東京科学大学
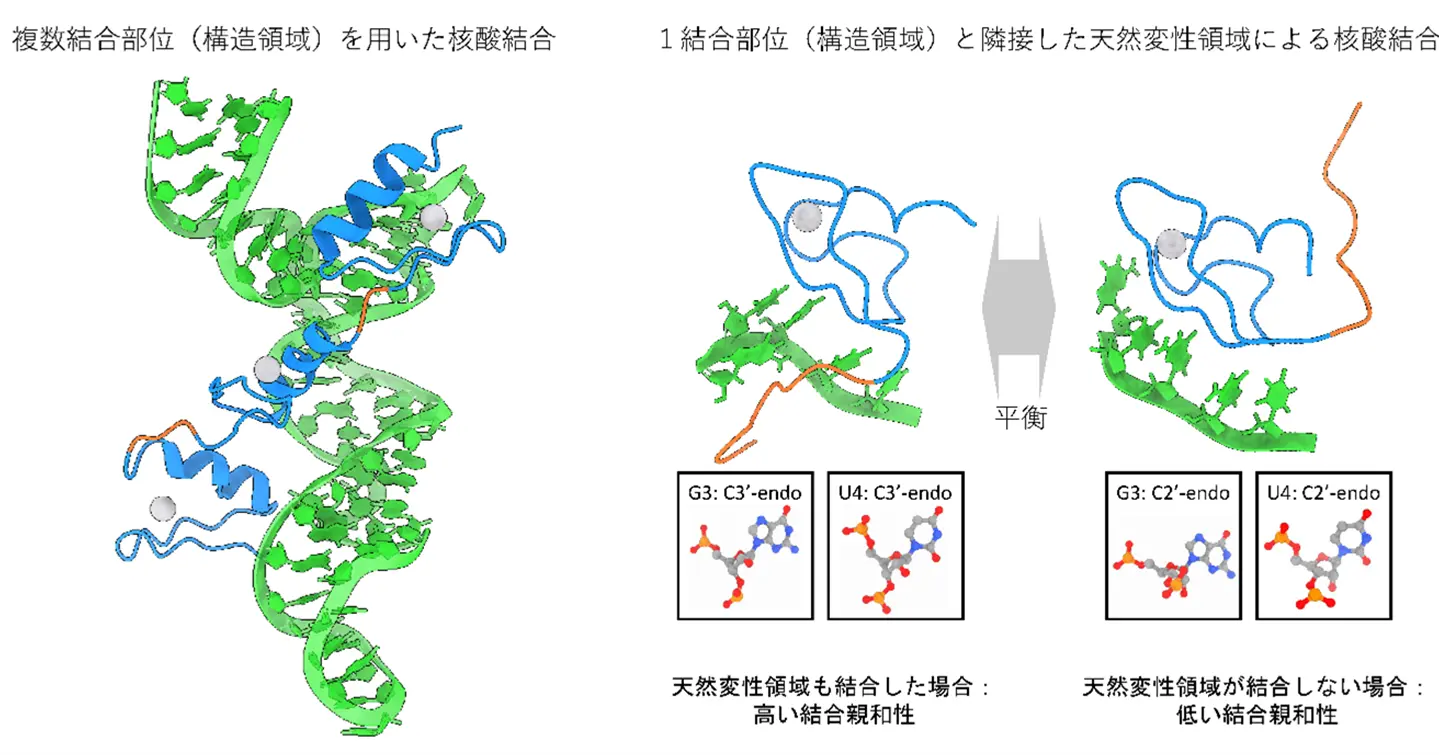 図1. 本研究で提示されたタンパク質による2種類の核酸認識機構
図1. 本研究で提示されたタンパク質による2種類の核酸認識機構
<関連情報>
- https://www.isct.ac.jp/ja/news/ty5ftupp2oa5
- https://www.isct.ac.jp/plugins/cms/component_download_file.php?type=2&pageId=&contentsId=1&contentsDataId=2119&prevId=&key=75896d69409e3587571d88d380244550.pdf
- https://pubs.acs.org/doi/10.1021/acs.jcim.5c01059
FUS亜鉛指のRNA結合メカニズムとその隣接する内在性無秩序領域との協調作用 RNA Binding Mechanism of the FUS Zinc Finger in Concert with Its Flanking Intrinsically Disordered Region
Soichiro Kijima,and Akio Kitao
Journal of Chemical Information and Modeling Published: July 22, 2025
DOI:https://doi.org/10.1021/acs.jcim.5c01059
Abstract
The zinc finger (ZnF) motif is one of the most common nucleic acid binding motifs in proteins and comprises approximately 5% of the human genome. Proteins often carry multiple copies of ZnF motifs, enhancing DNA- and RNA-binding affinity and specificity. Fused in Sarcoma (FUS) is an RNA-binding protein that contains one ZnF motif and one RNA recognition motif. We identified the molecular basis for the strong and specific binding of FUS ZnF to RNA at atomic resolution by performing molecular dynamics and dissociation parallel cascade selection molecular dynamics simulations of a single-stranded RNA complexed with FUS ZnF and the flanking RGG2 domain. The RGG2-ZnF construct was classified into two regions based on atomic fluctuation: an intrinsically disordered region (IDR) consisting mainly of RGG2 and a structured region consisting mainly of the ZnF motif. Our results on intermolecular interactions, dissociation process, binding affinity, and free energy landscape indicate that the structured region specifically recognizes the GGU sequence of RNA, while the IDR interacts nonspecifically with the RNA backbone and distorts it. Although the binding of the structured region alone with the short RNA sequence is relatively weak, the binding of the IDR exhibits a 2-fold lower dissociation constant due to the addition of nonspecific charge interactions with RNA backbone phosphate groups. Further comprehensive analysis of nucleic acid binding motifs in the DisProt database suggests that the stabilization of protein-RNA binding by only one or a few nucleic acid binding motifs with flanking disordered regions, as in the case of FUS, ZnF is a common mechanism for ensuring strong and specific nucleic acid binding in nucleic acid binding proteins, as is the involvement of more nucleic acid binding motifs. Our findings allow us to expand the repertoire of disordered region-assisted nucleic acid binding by ZnF from double-stranded DNAs to single-stranded RNAs.


