2025-12-10 分子科学研究所
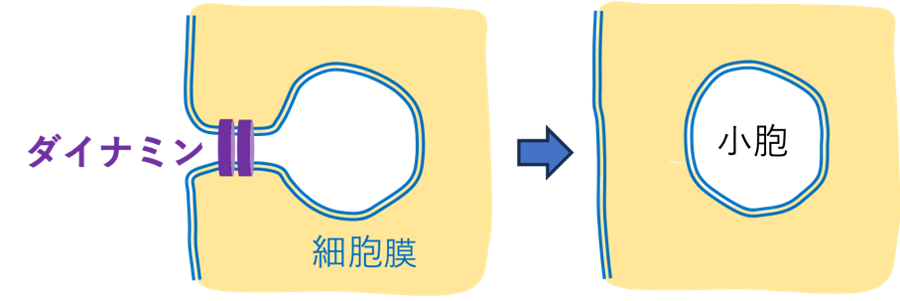
図1.エンドサイトーシス過程におけるダイナミンによる細胞膜切断
<関連情報>
粗視化分子動力学シミュレーションによるGTP駆動型構造変化を介したダイナミンによる膜収縮 Membrane Constriction by Dynamin through GTP-Driven Conformational Changes from Coarse-Grained Molecular Dynamics Simulations
Md. Iqbal Mahmood,Shintaroh Kubo,Hiroshi Noguchi,and Kei-ichi Okazaki
The Journal of Physical Chemistry Letters Published: November 20, 2025
DOI:https://doi.org/10.1021/acs.jpclett.5c02867
Abstract
Dynamin, a GTPase involved in membrane remodeling processes, plays a pivotal role in membrane fission during endocytosis. Understanding the molecular mechanisms of dynamin assembly and its conformational change triggered by GTP hydrolysis on lipid membranes is crucial for elucidating the intricate details of membrane constriction and fission events. However, the size and complexity of the dynamin assembly prevent us from obtaining comprehensive molecular mechanisms. In this study, we performed coarse-grained molecular dynamics (CG-MD) simulations to elucidate the chemo-mechanical coupling mechanism of dynamin membrane constriction. We set up a CG Martini simulation system that contains dynamin rings on a tubular membrane with realistic molecular structures. Controlling the length of the tubular membrane with a pressure coupling, we simulate membrane constriction through the nucleotide-state-dependent conformational change of dynamin. The simulation results suggest that the conformational change to the GDP state loosens and expands the dynamin rings, resulting in indirect membrane constriction in the protein-uncoated region. This study lays the groundwork for simulating the chemo-mechanical coupling of dynamin-mediated membrane constriction.