2026-01-30 東京科学大学
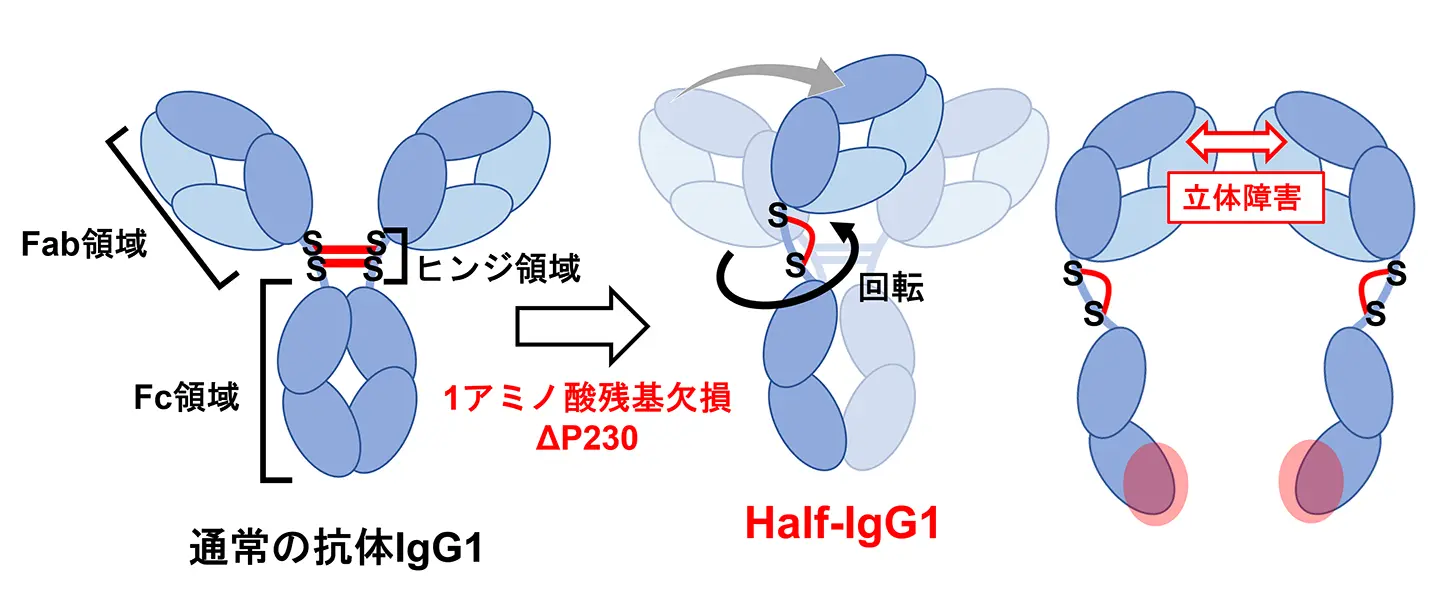
図1.半分子抗体が形成される分子メカニズム。通常の抗体IgG1のヒンジ領域を改変し(左)、半分子抗体Half-IgG1を作成した。このHalf-IgG1ではFab領域とFc領域が結合部で回転し、配置が変化している(中央)ため、立体障害が生じる(右)。
<関連情報>
- https://www.isct.ac.jp/ja/news/66zahymu6fuq
- https://www.isct.ac.jp/plugins/cms/component_download_file.php?type=2&pageId=&contentsId=1&contentsDataId=2988&prevId=&key=a58135d24878a68a3379741bd3586001.pdf
- https://pubs.acs.org/doi/10.1021/acs.jmedchem.5c02419
ヒンジ領域におけるPro230のIgG1の構造と機能における重要な役割 Key Role of Pro230 in the Hinge Region on the Architecture and Function of IgG1
Yuuki Koseki,Yuki Yamaguchi,Michihiko Aoyama,Kentaro Hiraka,Minoru Tada,Atsuji Kodama,Akinobu Senoo,Akiko Ishii-Watabe,Takayuki Uchihashi,Kazuyoshi Murata,Susumu Uchiyama,Koichi Kato,Saeko Yanaka,and Jose M.M. Caaveiro
Journal of medical Chemistry Published: January 29, 2026
DOI:https://doi.org/10.1021/acs.jmedchem.5c02419
Abstract
Immunoglobulin G (IgG) is a multifunctional glycoprotein essential for immune defense and widely used as a therapeutic due to its antigen specificity and effector functions. However, the inherent flexibility of its hinge region complicates structural characterization and obscures the molecular basis of its mechanism of action. To clarify the hinge’s role, we performed systematic amino acid substitutions. Notably, deletion of Pro230 led to the formation of a half-IgG1 species lacking inter-heavy chain interactions. Structural analysis using nuclear magnetic resonance (NMR), negative-stain EM, and disulfide bond quantification by LC-MS/MS peptide mapping revealed the mechanism underlying half-IgG1 generation. To enable this, we developed a new stable-isotope labeling method for NMR. Functional assays with FcγR-expressing reporter cells demonstrated that half-IgG1 retained selective FcγRI-mediated activity. These findings provide new insights into higher-order IgG structure and Fcγ receptor-dependent immune activation, offering a basis for designing next-generation antibody therapeutics.