子どもの遺伝性疾患における母親から受け継いだ遺伝子の役割を明らかにし、その理解を深めることができた The study highlights the role of genes inherited from mothers in genetic diseases in children, and improves the understanding of such diseases
2022-06-29 シンガポール国立大学(NUS)
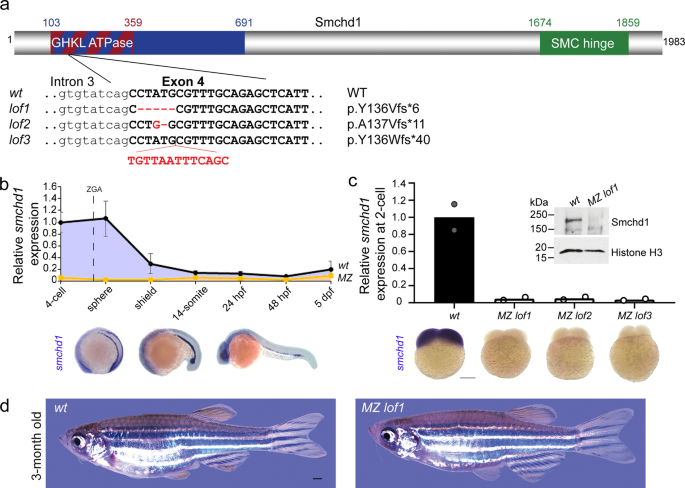
研究チームは、母親のSMCHD1が、胎児の頭から尾までの軸に沿った体の部位の位置を決めるHOX遺伝子と呼ばれる遺伝子群の発現を制御していることを発見した。また、メスのゼブラフィッシュでSMCHD1を不活性化すると、HOX遺伝子の発現が変化し、その子孫に骨格の欠損が生じることも明らかにした。
<関連情報>
- https://news.nus.edu.sg/bringing-new-light-to-unsolved-genetic-diseases/
- https://www.nature.com/articles/s41467-022-31185-8
母親のSMCHD1/LRIF1ハプロ不全によるHOXエピミューテーションは、遺伝的に野生型の子孫にホメオティックな形質転換を引き起こす。 HOX epimutations driven by maternal SMCHD1/LRIF1 haploinsufficiency trigger homeotic transformations in genetically wildtype offspring
Shifeng Xue,Thanh Thao Nguyen Ly,Raunak S. Vijayakar,Jingyi Chen,Joel Ng,Ajay S. Mathuru,Frederique Magdinier & Bruno Reversade
Nature Communications Published:23 June 2022
DOI:https://doi.org/10.1038/s41467-022-31185-8
Abstract
The body plan of animals is laid out by an evolutionary-conserved HOX code which is colinearly transcribed after zygotic genome activation (ZGA). Here we report that SMCHD1, a chromatin-modifying enzyme needed for X-inactivation in mammals, is maternally required for timely HOX expression. Using zebrafish and mouse Smchd1 knockout animals, we demonstrate that Smchd1 haplo-insufficiency brings about precocious and ectopic HOX transcription during oogenesis and embryogenesis. Unexpectedly, wild-type offspring born to heterozygous knockout zebrafish smchd1 mothers exhibited patent vertebrate patterning defects. The loss of maternal Smchd1 was accompanied by HOX epi-mutations driven by aberrant DNA methylation. We further show that this regulation is mediated by Lrif1, a direct interacting partner of Smchd1, whose knockout in zebrafish phenocopies that of Smchd1. Rather than being a short-lived maternal effect, HOX mis-regulation is stably inherited through cell divisions and persists in cultured fibroblasts derived from FSHD2 patients haploinsufficient for SMCHD1. We conclude that maternal SMCHD1/LRIF1 sets up an epigenetic state in the HOX loci that can only be reset in the germline. Such an unusual inter-generational inheritance, whereby a phenotype can be one generation removed from its genotype, casts a new light on how unresolved Mendelian diseases may be interpreted.