2023-02-22 ワシントン大学セントルイス校
◆機械工学・材料科学のアミット・パタック准教授は、生体医工学博士課程のカーリー・クルル、2022年にコンピューター科学・工学の学士号を取得したハイイ・リーと共同で、健康な細胞に抗がん剤レプトマイシンBを投与すると、細胞の成長は停止するが、核内の複数の競合遺伝子が活性化することを発見しました。この研究は、eLife誌に掲載されました。
<関連情報>
- https://source.wustl.edu/2023/02/cells-take-on-dual-identities/
- https://engineering.wustl.edu/news/2023/Cells-take-on-dual-identities-with-competing-factors-trapped-in-the-nucleus.html
- https://elifesciences.org/articles/81048
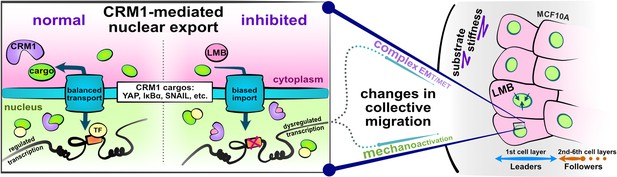
核輸出阻害は上皮間葉系を混乱させ、健康な上皮に移動性障害を生じさせる Nuclear export inhibition jumbles epithelial–mesenchymal states and gives rise to migratory disorder in healthy epithelia
Carly M Krull,Haiyi Li,Amit Pathak
eLife Published:Feb 21, 2023
DOI:https://doi.org/10.7554/eLife.81048
Abstract
Dynamic nucleocytoplasmic transport of E-M factors regulates cellular E-M states; yet, it remains unknown how simultaneously trapping these factors affects epithelia at the macroscale. To explore this question, we performed nuclear export inhibition (NEI) via leptomycin B and Selinexor treatment, which biases nuclear localization of CRM1-associated E-M factors. We examined changes in collective cellular phenotypes across a range of substrate stiffnesses. Following NEI, soft substrates elevate collective migration of MCF10A cells for up to 24 hr, while stiffer substrates reduce migration at all time points. Our results suggest that NEI disrupts migration through competition between intercellular adhesions and mechanoactivation, generally causing loss of cell–cell coordination. Specifically, across substrate stiffnesses, NEI fosters an atypical E-M state wherein MCF10A cells become both more epithelial and more mesenchymal. We observe that NEI fosters a range of these concurrent phenotypes, from more epithelial shYAP MCF10A cells to more mesenchymal MDCK II cells. α-Catenin emerges as a potential link between E-M states, where it maintains normal levels of intercellular adhesion and transmits mechanoactive characteristics to collective behavior. Ultimately, to accommodate the concurrent states observed here, we propose an expanded E-M model, which may help further understand fundamental biological phenomena and inform pathological treatments.
Editor’s evaluation
This work is an important contribution to our understanding of epithelial migration. Previous work had shown that nuclear export inhibition (NEI), which is employed as a therapeutic strategy to treat cancer, traps several known regulators of epithelial-mesenchymal (E-M) phenotypes; however, how NEI alters the mechano-response and collective cell migration of healthy epithelia on substrates of varying stiffness was not described. The convincing new results show that NEI induces an intermediate E-M state where cells concurrently strengthen intercellular adhesions and develop mechano-active characteristics. Migration of epithelial monolayers becomes disordered and leads to multicellular streaming.