2023-07-14 ペンシルベニア州立大学(PennState)
◆細菌は、細胞のシグナルと環境からの手がかりを使って、スクイッドに寄生する方法を調整している。このメカニズムは広範な細菌に広がっている可能性があり、細菌が宿主に寄生する際の細胞シグナルの調整を理解することは重要である。
◆研究は、eLife誌でオンラインで公開されている。研究者たちは、このメカニズムが細菌の広範な行動に広がっていると示唆しており、これは細菌がスクイッドに寄生する方法を理解する上で重要である。
<関連情報>
- https://www.psu.edu/news/eberly-college-science/story/bioluminescent-bacteria-coordinate-signaling-colonize-squids-light/
- https://elifesciences.org/articles/78544
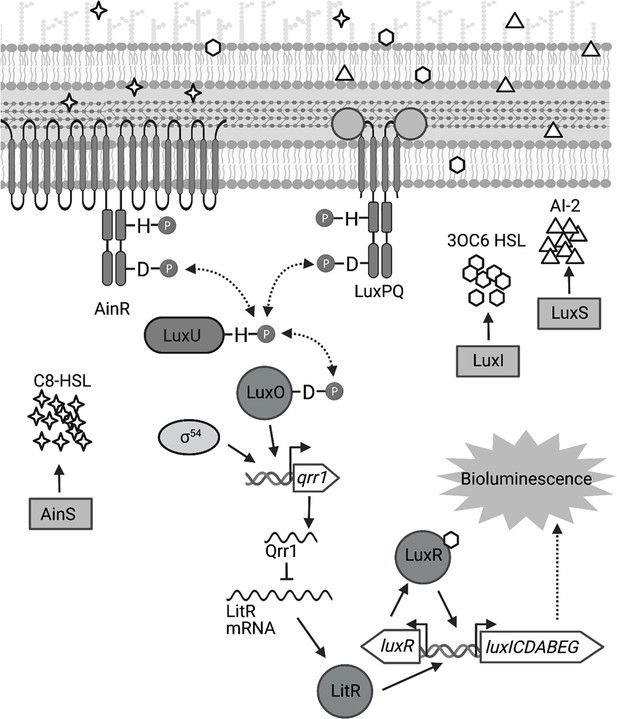
σ54に依存する細菌共生体の定足数制御RNAの転写を2つのエンハンサー結合タンパク質が活性化する Two enhancer binding proteins activate σ54-dependent transcription of a quorum regulatory RNA in a bacterial symbiont
Ericka D Surrett,Kirsten R Guckes,Shyan Cousins,Terry B Ruskoski,Andrew G Cecere,Denise A Ludvik,C Denise Okafor,Mark J Mandel,Tim I Miyashiro
eLife Published:May 5, 2023
DOI:https://doi.org/10.7554/eLife.78544
Abstract
To colonize a host, bacteria depend on an ensemble of signaling systems to convert information about the various environments encountered within the host into specific cellular activities. How these signaling systems coordinate transitions between cellular states in vivo remains poorly understood. To address this knowledge gap, we investigated how the bacterial symbiont Vibrio fischeri initially colonizes the light organ of the Hawaiian bobtail squid Euprymna scolopes. Previous work has shown that the small RNA Qrr1, which is a regulatory component of the quorum-sensing system in V. fischeri, promotes host colonization. Here, we report that transcriptional activation of Qrr1 is inhibited by the sensor kinase BinK, which suppresses cellular aggregation by V. fischeri prior to light organ entry. We show that Qrr1 expression depends on the alternative sigma factor σ54 and the transcription factors LuxO and SypG, which function similar to an OR logic gate, thereby ensuring Qrr1 is expressed during colonization. Finally, we provide evidence that this regulatory mechanism is widespread throughout the Vibrionaceae family. Together, our work reveals how coordination between the signaling pathways underlying aggregation and quorum-sensing promotes host colonization, which provides insight into how integration among signaling systems facilitates complex processes in bacteria.

