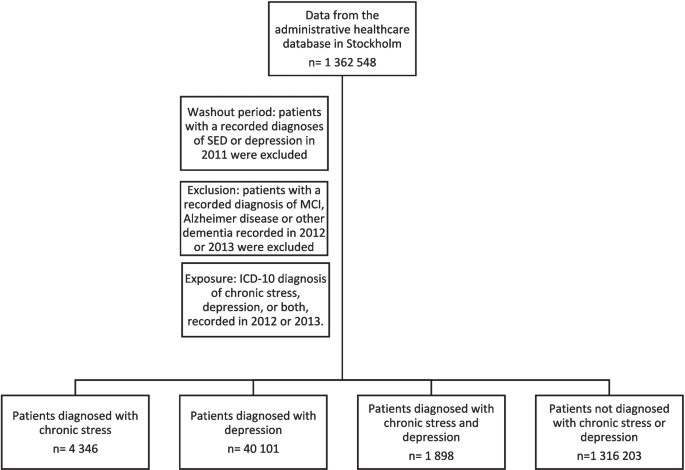
2023-09-29 カロリンスカ研究所(KI)

Photo: Getty Images
◆炎症性抗体やT細胞の機能にはほとんど変化が見られず、善玉菌による抗炎症反応もアレルギー免疫反応を抑制することができないようでした。この研究結果は、アレルギーのメカニズムに関する理解を深め、臨床的なアプローチに影響を与える可能性があります。
<関連情報>
- https://news.ki.se/allergy-study-on-wild-mice-challenges-the-hygiene-hypothesis
- https://www.science.org/doi/10.1126/sciimmunol.adf7702
野生の微生物叢を持つ実験用マウスが強いアレルギー免疫反応を起こす Laboratory mice with a wild microbiota generate strong allergic immune responses
Junjie Ma,Egon Urgard,Solveig Runge,Cajsa H. Classon,Laura Mathä,Julian M. Stark,Liqin Cheng,Javiera A. Álvarez,Silvia von Zedtwitz,Austeja Baleviciute,Sergio Martinez Hoyer,Muzhen Li,Anne Marleen Gernand,Lisa Osbelt,Agata Anna Bielecka,Till R. Lesker,Huey-Jy Huang,Susanne Vrtala,Louis Boon,Rudi Beyaert,Mikael Adner,Itziar Martinez Gonzalez,Till Strowig,Juan Du,Susanne Nylén,Stephan P. Rosshart,and Jonathan M. Coquet
Science Immunology Published:29 Sep 2023
DOI:https://doi.org/10.1126/sciimmunol.adf7702
Editor’s summary
The hygiene hypothesis proposes that early-life exposure to microbes protects against the development of allergies, a possible explanation for the alarming rise in allergic disorders in developed countries. However, a causal link between early-life infections and allergies is lacking. To uncover the impact of lifelong microbial exposure on the development of allergic inflammation, Ma and Urgard et al. compared experimental wildling mice, which have a representative breadth of naturally occurring pathogens, to specific pathogen–free mice. Surprisingly, when challenged with allergens, wildlings developed robust signs of pathological inflammation and allergic responses, with rapid expansion of TH2 cells in the lungs. The study identifies that increased microbial biodiversity in wildlings did not protect against allergic inflammation, in contrast to what would be predicted by the hygiene hypothesis. —Hannah Isles
Abstract
Allergic disorders are caused by a combination of hereditary and environmental factors. The hygiene hypothesis postulates that early-life microbial exposures impede the development of subsequent allergic disease. Recently developed “wildling” mice are genetically identical to standard laboratory specific pathogen–free (SPF) mice but are housed under seminatural conditions and have rich microbial exposures from birth. Thus, by comparing conventional SPF mice with wildlings, we can uncouple the impact of lifelong microbial exposures from genetic factors on the allergic immune response. We found that wildlings developed larger populations of antigen-experienced T cells than conventional SPF mice, which included interleukin-10–producing CD4 T cells specific for commensal Lactobacilli strains and allergy-promoting T helper 2 (TH2) cells. In models of airway exposure to house dust mite (HDM), recombinant interleukin-33, or Alternaria alternata, wildlings developed strong allergic inflammation, characterized by eosinophil recruitment, goblet cell metaplasia, and antigen-specific immunoglobulin G1 (IgG1) and IgE responses. Wildlings developed robust de novo TH2 cell responses to incoming allergens, whereas preexisting TH2 cells could also be recruited into the allergic immune response in a cytokine-driven and TCR-independent fashion. Thus, wildling mice, which experience diverse and lifelong microbial exposures, were not protected from developing pathological allergic immune responses. Instead, wildlings mounted robust allergic responses to incoming allergens, shedding new light on the hygiene hypothesis.