2024-01-29 ペンシルベニア州立大学(PennState)
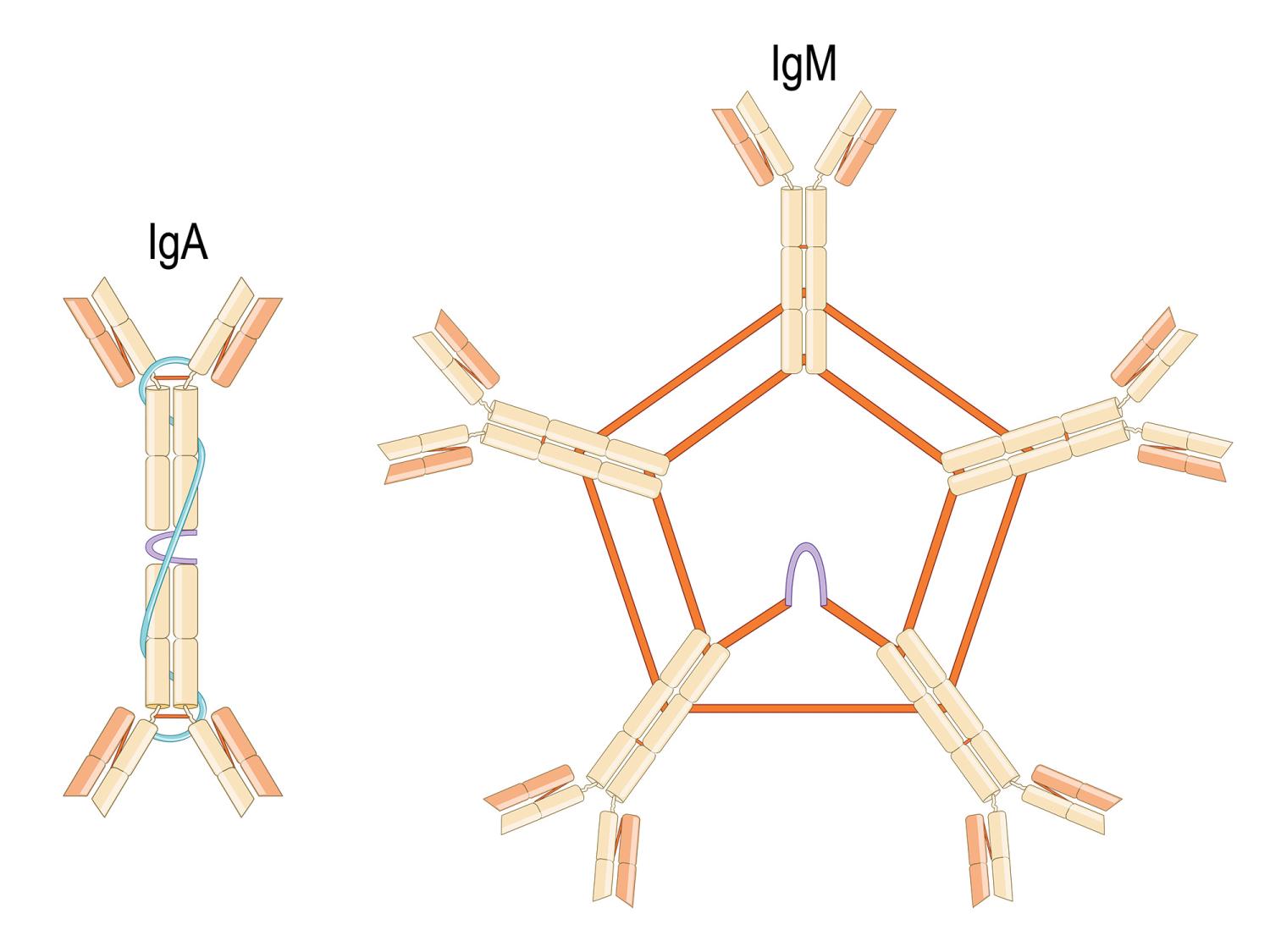
The Joining chain (J chain) that helps bind and regulate the subunits that make up antibodies was evolutionarily co-opted by the human immune system from another biological process, according to a study by researchers from Penn State, the University of Maryland and the University of Chicago. The J chain, shown here in purple, assembles and stabilizes, from left, immunoglobin A (IgA) and immunoglobin M (IgM) antibodies. It is also required to move IgA and IgM across the mucus-producing tissue lining body structures with external exposure, like the intestine, nasal cavity and lungs. Credit: Ttsz/Getty Images. All Rights Reserved.
◆J鎖と呼ばれるこのタンパク質は、免疫グロブリンMおよび免疫グロブリンAの構造を安定化させ、これらの分子が体内を移動するのを助ける重要な役割を果たしている。J鎖は、白血球の移動を調節する特定のタンパク質ファミリーから進化的に派生したことが判明した。
◆これにより、免疫システムの基本理解に寄与するだけでなく、将来の個別化された免疫応答などの治療法の可能性を開く手がかりとなる可能性がある。
<関連情報>
- https://www.psu.edu/news/research/story/evolutionary-origin-mysterious-immune-system-molecule-humans-revealed/
- https://www.pnas.org/doi/abs/10.1073/pnas.2318995121
免疫グロブリンJ鎖は進化的にケモカインと共役である The immunoglobulin J chain is an evolutionarily co-opted chemokine
Kazuhiko Kawasaki, Yuko Ohta, Caitlin D. Castro, and Martin F. Flajnik
Proceedings of the National Academy of Sciences Published:January 12, 2024
DOI:https://doi.org/10.1073/pnas.2318995121
Abstract
The joining (J) chain regulates polymerization of multimeric Immunoglobulin(Ig)M and IgA, forming a disulfide bond to the C termini of their Ig heavy chains, and it controls IgM/IgA transport across mucosal epithelia. Like Ig itself and human-like adaptive immunity, J chain emerged in jawed vertebrates (gnathostomes), but its origin has remained mysterious since its discovery over 50 y ago. Here, we show unexpectedly that J chain is a member of the CXCL chemokine family. The J chain gene (JCHAIN) is linked to clustered CXCL chemokine loci in all gnathostomes except actinopterygians that lost JCHAIN. JCHAIN and most CXCL genes have four exons with the same intron phases, including the same cleavage site for the signal peptide/mature protein. The second exon of both genes encodes a CXC motif at the same position, and the lengths of exons 1 to 3 are similar. No other gene in the human secretome shares all of these characteristics. In contrast, intrachain disulfide bonds of the two proteins are completely different, likely due to modifications in J chain to direct Ig polymerization and mucosal transport. Crystal structures of CXCL8 and J chain share a conserved beta-strand core but diverge otherwise due to different intrachain disulfide bonds and extension of the J chain C terminus. Identification of this ancestral affiliation between J chain and CXCL chemokines addresses an age-old problem in immunology.