2024-07-08 米国国立標準技術研究所(NIST)
<関連情報>
- https://www.nist.gov/news-events/news/2024/07/nists-new-hemp-reference-material-will-help-ensure-accurate-cannabis
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10924369/
カンナビノイド含有量の正確な表示に関する薬局の大麻花の評価 Evaluation of dispensaries’ cannabis flowers for accuracy of labeling of cannabinoids content
Mona M. Geweda, Chandrani G. Majumdar, Malorie N. Moore, Mostafa A. Elhendawy,, Mohamed M. Radwan, Suman Chandra, and Mahmoud A. ElSohly
Journal of Cannabis Research Published:2024 Mar 9
DOI:https://doi.org/10.1186%2Fs42238-024-00220-4
Abstract
Background
Cannabis policies have changed drastically over the last few years with many states enacting medical cannabis laws, and some authorizing recreational use; all against federal laws. As a result, cannabis products are marketed in dispensaries in different forms, most abundantly as flowers intended for smoking and sometimes vaping. All samples used in this study were obtained directly from law enforcement. The sample collection process was facilitated and funded by the National Marijuana Initiative (NMI), part of the High-Intensity Drug Trafficking Area (HIDTA) program. This initial report focuses on cannabis flowers. Similar studies with other cannabis products will be the subject of a future report.
Methods
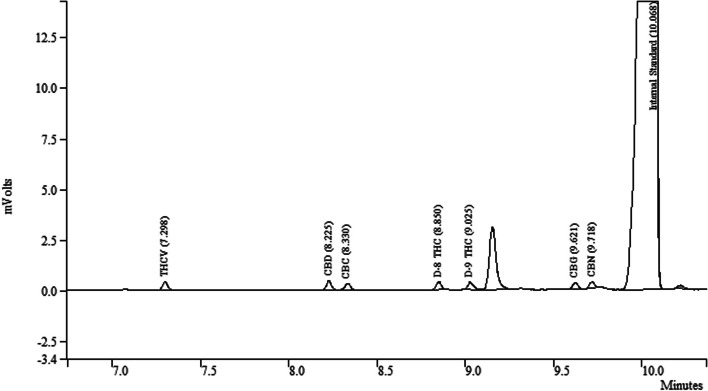
A total of 107 Δ9-THC cannabis flower samples were collected by law enforcement from adult commercial use cannabis dispensaries, located in three different states (Colorado, Oregon, and California) and analyzed in this study for cannabinoid concentration. Samples were analyzed by GC-FID following our previously published procedure.
Discussion
The label claims for total Δ9-THC content ranged from 12.04 to 58.20% w/w, while GC-FID results showed a concentration ranging from 12.95 to 36.55% w/w. Of the evaluated 107 products, only 32 samples have Δ9-THC content within ± 20% of the labeled content. However, the remaining 75 samples were found to be out of the ± 20% acceptance criteria. The degree of agreement for the tested samples using ± 20% tolerance with label claims was only 30%. The results of this study indicate that there is a need for more stringent regulations to ensure that product labeling is accurate, as 70% of the evaluated products did not meet the ± 20% acceptance criteria. This highlights the importance of healthcare professionals and patients being vigilant about the Δ9-THC content, as inaccurate labeling of cannabis products could potentially result in adverse health effects. Furthermore, there is a pressing need for more rigorous regulation of commercial cannabis products in the United States.
Supplementary Information
The online version contains supplementary material available at 10.1186/s42238-024-00220-4.