2025-03-28 京都大学
ChatGPT:
<関連情報>
- https://www.kyoto-u.ac.jp/ja/research-news/2025-03-28
- https://www.kyoto-u.ac.jp/sites/default/files/2025-03/web_2503_Nomura-e0620dbff9a217c48a92f5ef13d0a737.pdf
- https://www.nature.com/articles/s41586-025-08773-x
ウイルス膜タンパク質を標的とするコロナウイルス集合阻害剤 A coronavirus assembly inhibitor that targets the viral membrane protein
Manon Laporte,Dirk Jochmans,Dorothée Bardiot,Lowiese Desmarets,Oliver J. Debski-Antoniak,Giulia Mizzon,Rana Abdelnabi,Pieter Leyssen,Winston Chiu,Zhikuan Zhang,Norimichi Nomura,Sandro Boland,Umeharu Ohto,Yannick Stahl,Jurgen Wuyts,Steven De Jonghe,Annelies Stevaert,Martijn J. van Hemert,Brenda W. Bontes,Patrick Wanningen,G. J. Mirjam Groenewold,Aneta Zegar,Katarzyna Owczarek,Sanjata Joshi,… Johan Neyts
Nature Published:26 March 2025
DOI:https://doi.org/10.1038/s41586-025-08773-x
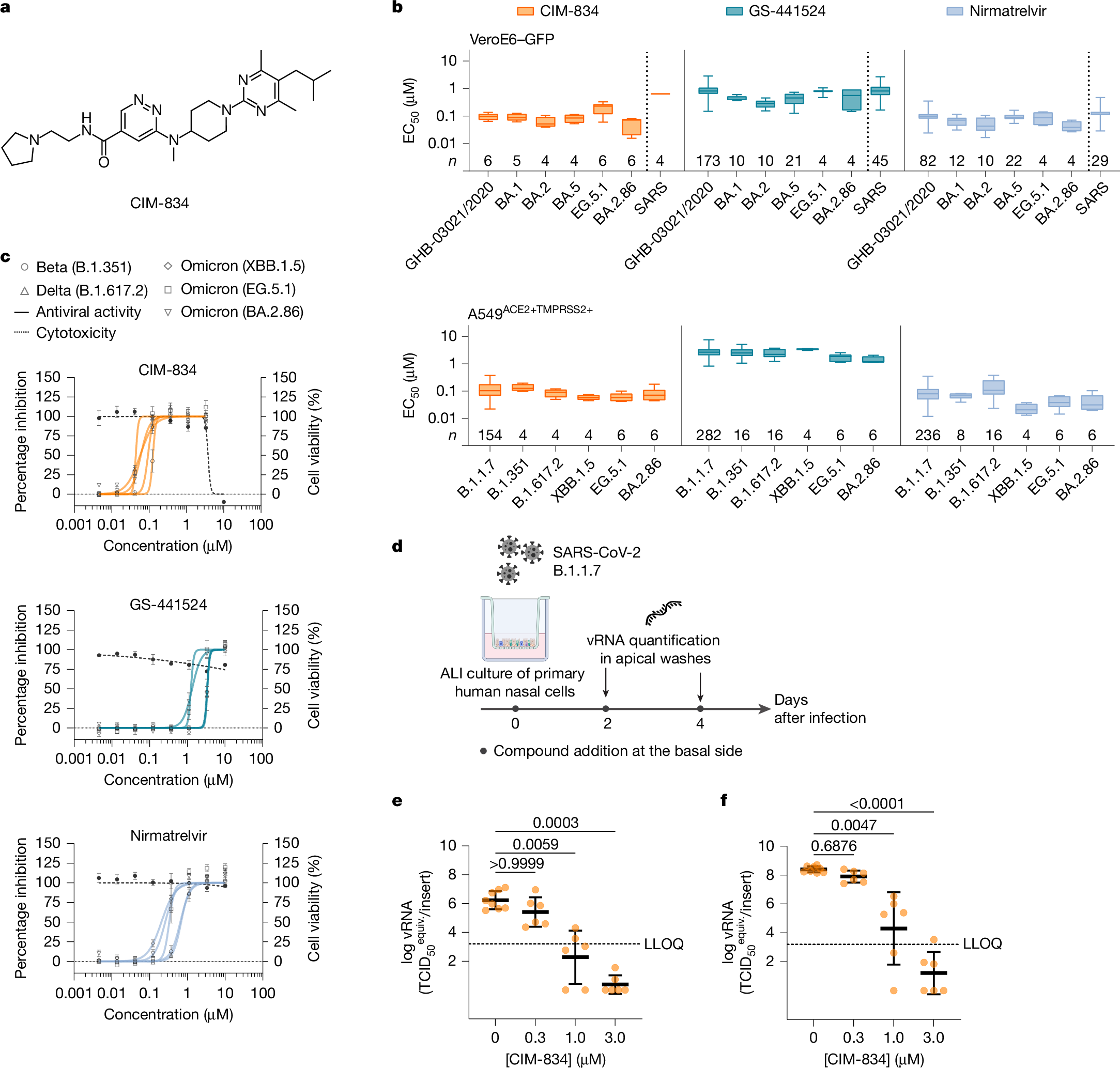
Abstract
The coronavirus membrane protein (M) is the main organizer of coronavirus assembly. Here, we report on an M-targeting molecule, CIM-834, that blocks the assembly of SARS-CoV-2. CIM-834 was obtained through high-throughput phenotypic antiviral screening followed by medicinal-chemistry efforts and target elucidation. CIM-834 inhibits the replication of SARS-CoV-2 (including a broad panel of variants) and SARS-CoV. In SCID mice and Syrian hamsters intranasally infected with SARS-CoV-2, oral treatment reduced lung viral titres to nearly undetectable levels, even (as shown in mice) when treatment was delayed until 24 h before the end point. Treatment of infected hamsters prevented transmission to untreated sentinels. Transmission electron microscopy studies show that virion assembly is completely absent in cells treated with CIM-834. Single-particle cryo-electron microscopy reveals that CIM-834 binds and stabilizes the M protein in its short form, thereby preventing the conformational switch to the long form, which is required for successful particle assembly. In conclusion, we have discovered a new druggable target in the replication cycle of coronaviruses and a small molecule that potently inhibits it.