2025-04-01 アメリカ国立衛生研究所(NIH)
米国国立衛生研究所(NIH)の研究者らは、転移性消化器がん患者に対する新しい腫瘍浸潤リンパ球(TIL)療法の臨床試験を実施し、結腸、直腸、膵臓、胆管などの腫瘍に対して有望な効果を確認しました。
◆このTIL療法は、患者自身の腫瘍から免疫細胞を抽出・増殖させ、再び体内に戻す個別化免疫療法です。従来のTIL療法に比べ、特定の腫瘍抗原を認識するリンパ球を選択的に増殖させることで、治療効果を向上させています。
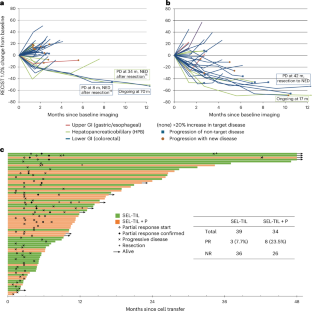
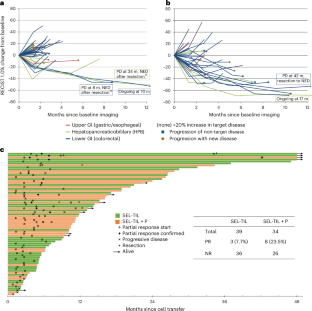
◆試験では、治療を受けた患者の一部で腫瘍の縮小が確認され、特に直腸がん患者では肝転移が顕著に減少しました。この成果は、これまで細胞療法の効果が限定的だった固形腫瘍に対する新たな治療法の可能性を示しています。
◆研究者らは、今後さらなる臨床試験を通じて、このTIL療法の有効性と安全性を検証し、転移性消化器がん患者への適用拡大を目指しています。
<関連情報>
- https://www.nih.gov/news-events/news-releases/combination-immunotherapy-shrank-variety-metastatic-gastrointestinal-cancers
- https://www.nature.com/articles/s41591-025-03627-5
消化器癌における新抗原特異的腫瘍浸潤リンパ球:第2相試験 Neoantigen-specific tumor-infiltrating lymphocytes in gastrointestinal cancers: a phase 2 trial
Frank J. Lowery,Stephanie L. Goff,Billel Gasmi,Maria R. Parkhurst,Nivedita M. Ratnam,Hyunmi K. Halas,Thomas E. Shelton,Michelle M. Langhan,Aarushi Bhasin,Aaron J. Dinerman,Victoria Dulemba,Ian S. Goldlust,Alexandra M. Gustafson,Abraham A. Hakim,Kyle J. Hitscherich,Lisa M. Kenney,Lior Levy,Juliette G. Rault-Wang,Alakesh Bera,Satyajit Ray,Courtney D. Seavey,Chuong D. Hoang,Jonathan M. Hernandez,Jared J. Gartner,… Steven A. Rosenberg
Nature Medicine Published:01 April 2025
DOI:https://doi.org/10.1038/s41591-025-03627-5
Abstract
Adoptive transfer of unselected autologous tumor-infiltrating lymphocytes (TILs) has mediated meaningful clinical responses in patients with metastatic melanoma but not in cancers of gastrointestinal epithelial origin. In an evolving single-arm phase 2 trial design, TILs were derived from and administered to 91 patients with treatment-refractory mismatch repair proficient metastatic gastrointestinal cancers in a schema with lymphodepleting chemotherapy and high-dose interleukin-2 (three cohorts of an ongoing trial). The primary endpoint of this study was the objective response rate as measured using Response Evaluation Criteria in Solid Tumors 1.0; safety was a descriptive secondary endpoint. In the pilot phase, no clinical responses were observed in 18 patients to bulk, unselected TILs; however, when TILs were screened and selected for neoantigen recognition (SEL-TIL), three responses were seen in 39 patients (7.7% (95% confidence interval (CI): 2.7–20.3)). Based on the high levels of programmed cell death protein 1 in the infused TILs, pembrolizumab was added to the regimen (SEL-TIL + P), and eight objective responses were seen in 34 patients (23.5% (95% CI: 12.4–40.0)). All patients experienced transient severe hematologic toxicities from chemotherapy. Seven (10%) patients required critical care support. Exploratory analyses for laboratory and clinical correlates of response were performed for the SEL-TIL and SEL-TIL + P treatment arms. Response was associated with recognition of an increased number of targeted neoantigens and an increased number of administered CD4+ neoantigen-reactive TILs. The current strategy (SEL-TIL + P) exceeded the parameters of the trial design for patients with colorectal cancer, and an expansion phase is accruing. These results could potentially provide a cell-based treatment in a population not traditionally expected to respond to immunotherapy. ClinicalTrials.gov identifier: NCT01174121.