2025-04-18 東京大学
<関連情報>
- https://www.t.u-tokyo.ac.jp/press/pr2025-04-18-001
- https://www.t.u-tokyo.ac.jp/hubfs/press-release/2025/0418/001/text.pdf
- https://www.nature.com/articles/s41467-025-58538-3
ミトコンドリアの複雑性はPDZD8-FKBP8テザリングを介してER-ミトコンドリア接触部位で制御されている Mitochondrial complexity is regulated at ER-mitochondria contact sites via PDZD8-FKBP8 tethering
Koki Nakamura,Saeko Aoyama-Ishiwatari,Takahiro Nagao,Mohammadreza Paaran,Christopher J. Obara,Yui Sakurai-Saito,Jake Johnston,Yudan Du,Shogo Suga,Masafumi Tsuboi,Makoto Nakakido,Kouhei Tsumoto,Yusuke Kishi,Yukiko Gotoh,Chulhwan Kwak,Hyun-Woo Rhee,Jeong Kon Seo,Hidetaka Kosako,Clint Potter,Bridget Carragher,Jennifer Lippincott-Schwartz,Franck Polleux & Yusuke Hirabayashi
Nature Communications Published:17 April 2025
DOI:https://doi.org/10.1038/s41467-025-58538-3
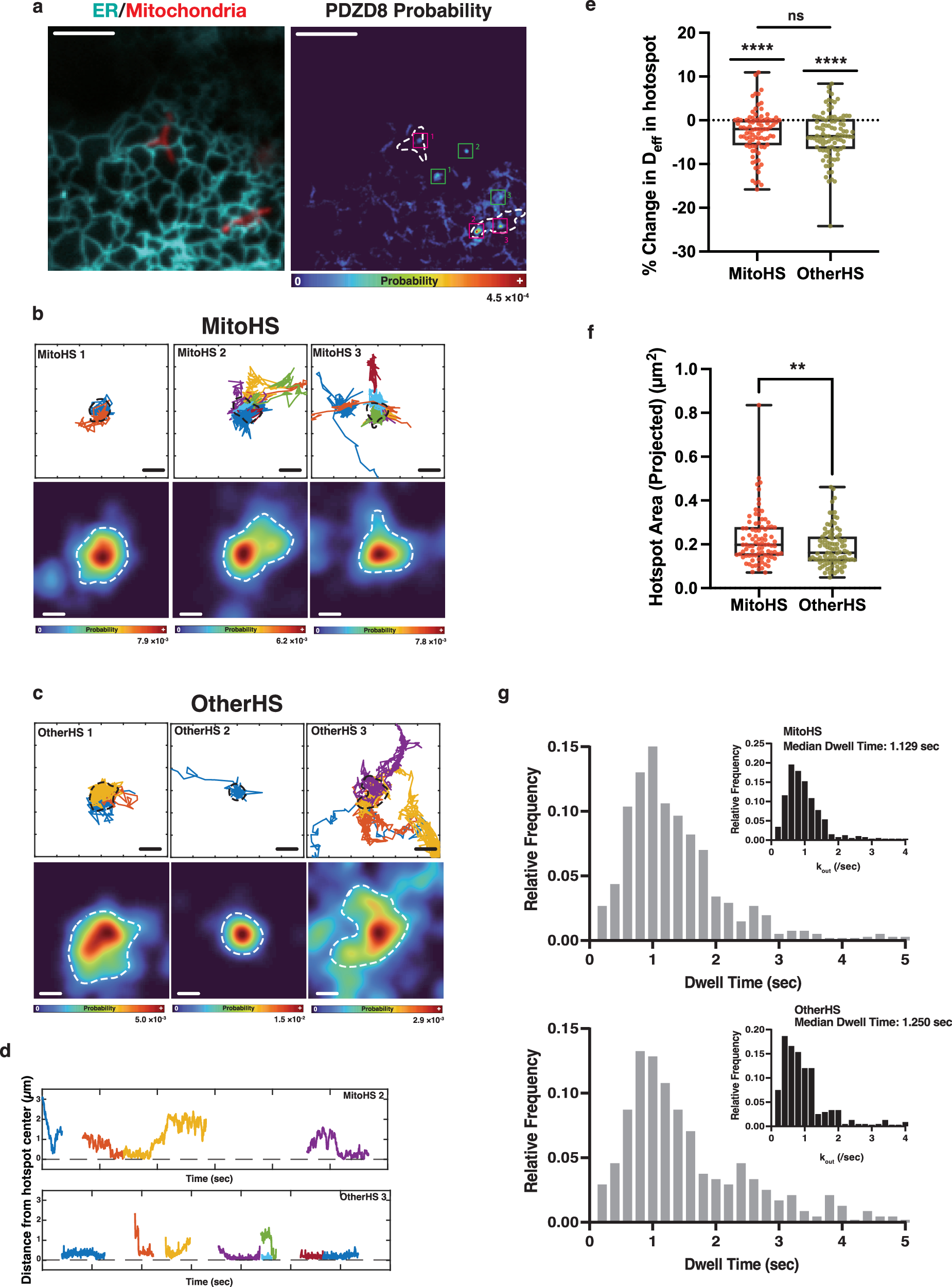
Abstract
Mitochondria-ER membrane contact sites (MERCS) represent a fundamental ultrastructural feature underlying unique biochemistry and physiology in eukaryotic cells. The ER protein PDZD8 is required for the formation of MERCS in many cell types, however, its tethering partner on the outer mitochondrial membrane (OMM) is currently unknown. Here we identify the OMM protein FKBP8 as the tethering partner of PDZD8 using a combination of unbiased proximity proteomics, CRISPR-Cas9 endogenous protein tagging, Cryo-electron tomography, and correlative light-electron microscopy. Single molecule tracking reveals highly dynamic diffusion properties of PDZD8 along the ER membrane with significant pauses and captures at MERCS. Overexpression of FKBP8 is sufficient to narrow the ER-OMM distance, whereas independent versus combined deletions of these two proteins demonstrate their interdependence for MERCS formation. Furthermore, PDZD8 enhances mitochondrial complexity in a FKBP8-dependent manner. Our results identify a novel ER-mitochondria tethering complex that regulates mitochondrial morphology in mammalian cells.


