2025-06-09 九州大学
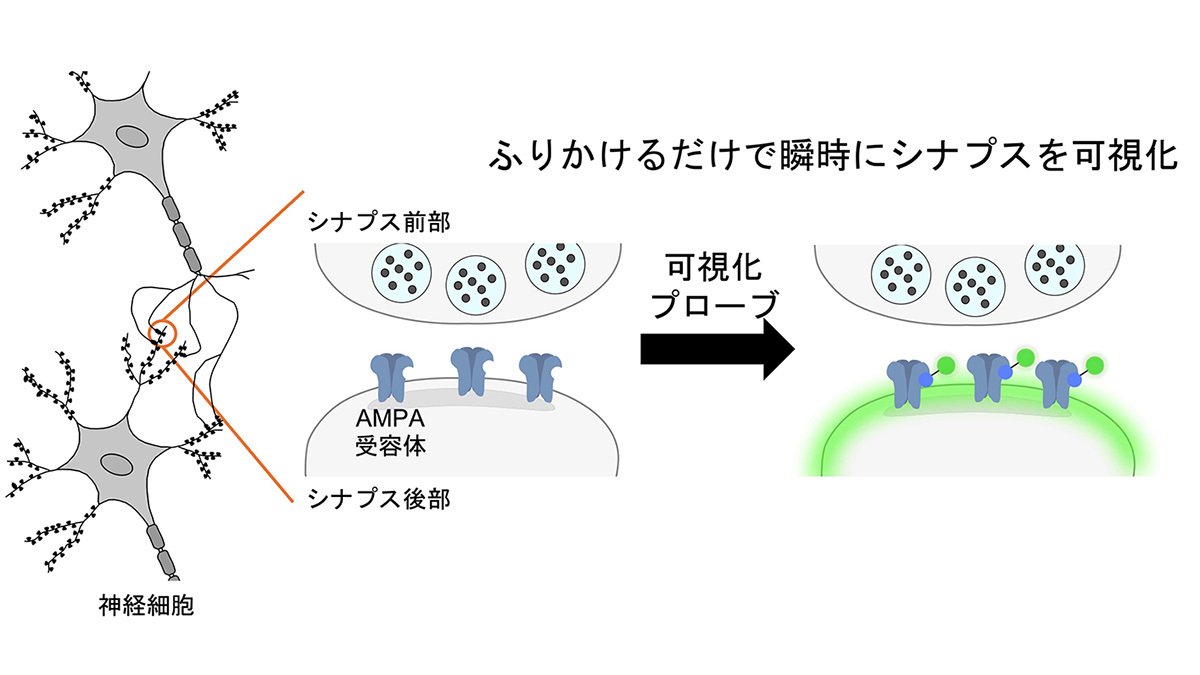
図1 本研究の概要図 本研究に関わる背景と主な実験結果を示す
<関連情報>
- https://www.kyushu-u.ac.jp/ja/researches/view/1280
- https://www.kyushu-u.ac.jp/f/61918/25_0609_02.pdf
- https://www.jbc.org/article/S0021-9258(25)02158-1/fulltext
マウス卵母細胞中期II期におけるH3K4me3の特性解析 Characterization of H3K4me3 in mouse oocytes at the metaphase II stage
Atsushi Takasu ∙ Toshiaki Hino ∙ Osamu Takenouchi ∙ … ∙ Kazuya Matsumoto ∙ Tomoya S. Kitajima ∙ Kei Miyamoto
Journal of Biological Chemistry Published:May 29, 2025
DOI:https://doi.org/10.1016/j.jbc.2025.110308
Abstract
Central functions of histone modifications in germ cell and embryonic development have been documented. Accumulating evidence suggests that oocytes possess unique profiles of histone modifications, among which histone H3 lysine 4 trimethylation (H3K4me3) is broadly spread on the mouse oocyte chromosomes at the metaphase II (MII) stage, unlike later embryonic stages. However, the characteristics and developmental roles of H3K4me3 on MII chromosomes are unclear. Here, we discovered that H3K4me3 was abundantly localized on some of the MII oocyte chromosomes facing the cortical side. Using multicolor FISH and CRISPR-Sirius-based labeling of chromosomes, we revealed that the X chromosome tended to be localized at the cortical side with strong H3K4me3 signals. Anchoring oocyte chromosomes to the cortex may play a role in the asymmetric H3K4me3 distribution. Furthermore, we found that the forced removal of H3K4me3 through the overexpression of a specific lysine demethylase in MII oocytes resulted in abnormal chromosome-spindle structure and impaired preimplantation development after in vitro fertilization. These findings highlight the developmental function of H3K4me3 in transcriptionally-silent MII oocytes.