2025-06-13 分子科学研究所
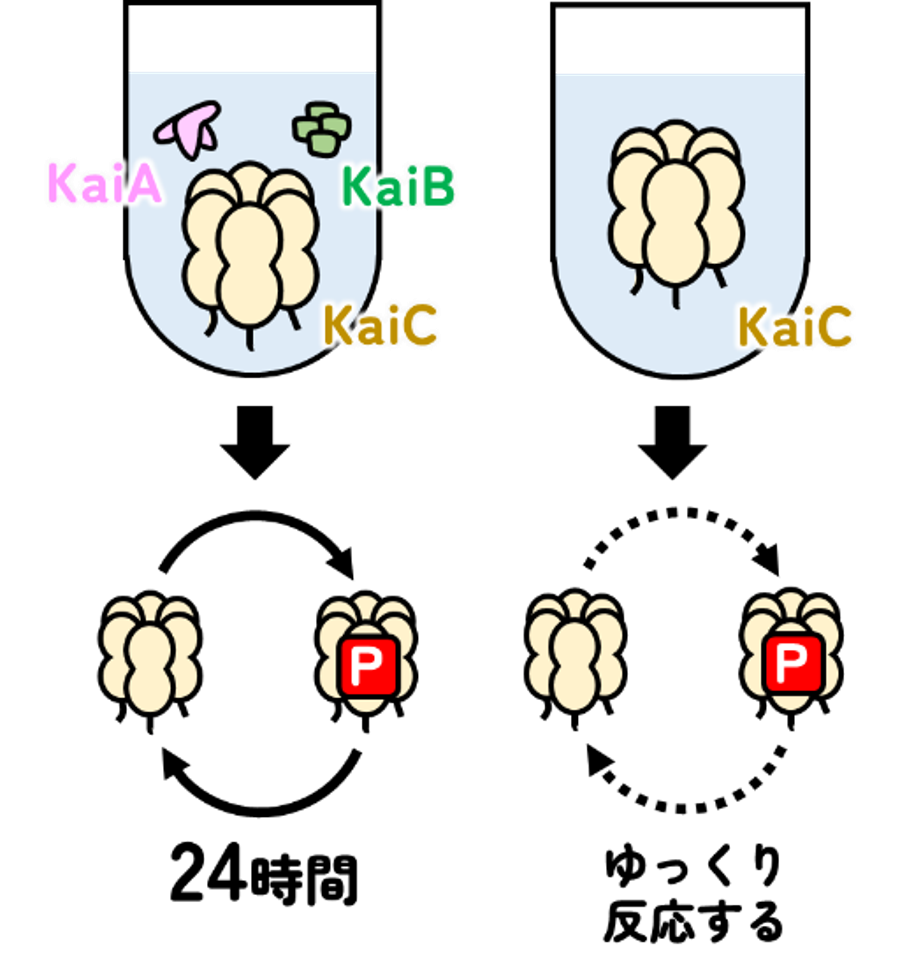 図1:時計タンパク質KaiCのリン酸化と脱リン酸化、Pはリン酸を表す
図1:時計タンパク質KaiCのリン酸化と脱リン酸化、Pはリン酸を表す
<関連情報>
- https://www.ims.ac.jp/news/2025/06/0613.html
- https://academic.oup.com/pnasnexus/article/4/5/pgaf136/8120782?login=false
The priming phosphorylation of KaiC is activated by the release of its autokinase autoinhibition 時計タンパク質KaiCのリン酸化は自己阻害メカニズムによって制御される
Yoshihiko Furuike , Yasuhiro Onoue , Shinji Saito , Toshifumi Mori , Shuji Akiyama
PNAS Nexus Published:28 April 2025
DOI:https://doi.org/10.1093/pnasnexus/pgaf136
Abstract
KaiC, a cyanobacterial circadian clock protein with autokinase activity, catalyzes the dual phosphorylation of its own S431 and T432 residues in a circadian manner in the presence of KaiA and KaiB. Priming phosphorylation at T432 is a key step that promotes secondary phosphorylation at S431. Although KaiA binding is considered essential for KaiC phosphorylation, the mechanisms underlying the activation and inactivation of priming phosphorylation remain elusive. We found that although the priming phosphorylation is autoinhibited within KaiC, it actually proceeds at a rate constant of 0.019 h-1 even in the absence of KaiA. The autoinhibition of KaiC and the mechanism underlying the release from autoinhibition by KaiA were examined by KaiC structural analysis and by classical molecular dynamics and quantum mechanics/molecular mechanics simulations. We found that the side chain of T432 adopts two rotamers in dephosphorylated KaiC, one of which places T432 in a position suitable for a nucleophilic attack on the terminal phosphate of adenosine triphosphate. However, the nucleophilicity of T432 was insufficient to overcome an energy barrier of ∼21 kcal mol-1 because the catalytic function of a nearby base, E318, was self-suppressed by hydrogen bonding to positively charged R385. Biochemical assays of KaiC mutants showed that the autoinhibition of KaiC autokinase activity is attenuated by conferring T432 high nucleophilicity through the KaiA-assisted release of R385 from E318 to E352. During the circadian cycle, R385 switches interacting partners to inactivate/activate the autokinase function and to ensure the unidirectionality of the KaiC phosphorylation cycle.