2025-06-26 東京大学
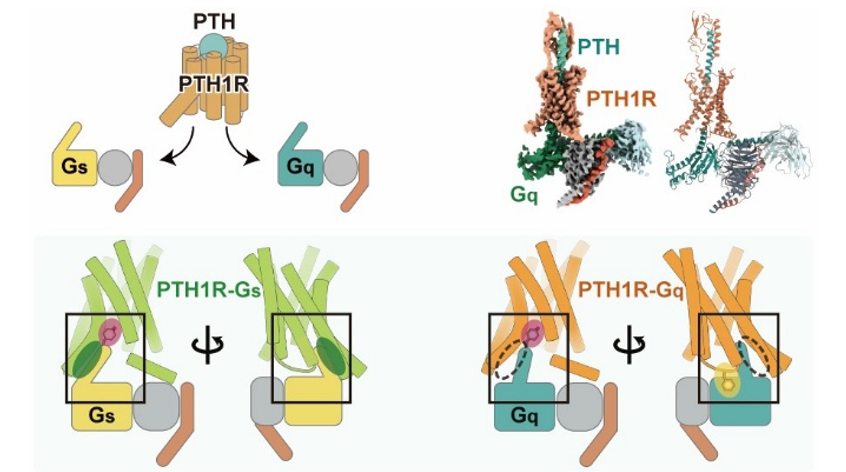
立体構造から明らかになるPTH1RのGタンパク質選択機構
<関連情報>
Gq結合PTH1Rの低温電子顕微鏡構造からGタンパク質カップリングの嗜好性が明らかになった Insights into G-protein coupling preference from cryo-EM structures of Gq-bound PTH1R
Fumiya K. Sano,Kota Shimizume,Kazuhiro Kobayashi,Toshikuni Awazu,Kouki Kawakami,Hiroaki Akasaka,Takaaki A. Kobayashi,Tatsuki Tanaka,Hiroyuki H. Okamoto,Hisato Hirano,Tsukasa Kusakizako,Wataru Shihoya,Yoshiaki Kise,Yuzuru Itoh,Ryuichiro Ishitani,Yasushi Okada,Yasushi Sako,Masataka Yanagawa,Asuka Inoue & Osamu Nureki
Nature Chemical Biology Published:26 June 2025
DOI:https://doi.org/10.1038/s41589-025-01957-6
Abstract
The parathyroid hormone type 1 receptor (PTH1R) is a prototypical class B1 G-protein-coupled receptor that couples to both Gq and Gs, having a crucial role in calcium homeostasis and serving as a therapeutic target for osteoporosis. Therapies targeting PTH1R face challenges because of Gq-associated prolonged signaling, which leads to bone resorption. To address this, selective activation of Gs signaling is desirable. However, the structural basis of Gq-mediated signaling remains unclear, limiting the development of signal-selective drugs. Here, we present cryo-electron microscopy structures of the PTH1R–Gq complex in two distinct extracellular conformations, demonstrating the role of N-linked glycans at N1761.28 in stabilizing the ligand-tilted conformation. Comparison with a Gs-bound PTH1R structure highlights the role of key interactions involving both the C terminus of Gα and the receptor’s intracellular loop 2 in Gq signaling. These structural insights provide a foundation for understanding the molecular mechanisms of PTH1R signaling.

