2025-10-18 京都産業大学
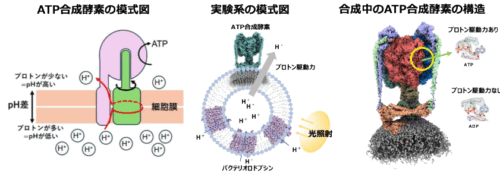
図 1. (左)ATP 合成酵素の模式図。膜の片側のプロトン濃度が高いと、プロトン駆動力が発生し、固定子(ピンク)と回転子(緑)の間をプロトンが通過することで、回転子が固定子に対して回転する。(中)本研究で用いた実験系。リポソームに ATP 合成酵素とバクテリオロドプシンを同時に再構成する。光を当てることで、バクテリオロドプシンがリポソーム内部にプロトンを輸送する。内部のプロトン濃度が高まることで外側へのプロトン駆動力が発生する。(右)ATP 合成中の ATP 合成酵素の構造。プロトン駆動力が存在すると触媒部位に合成された ATP が確認できた。
<関連情報>
- https://www.kyoto-su.ac.jp/news/news-001831.html
- https://www.kyoto-su.ac.jp/mt_uploads/20251018_press01.pdf
- https://www.science.org/doi/10.1126/sciadv.adx8771
プロトン駆動力で ATP 合成している好熱菌由来 ATP 合成酵素の構造 Structures of rotary ATP synthase from Thermus thermophilus during proton powered ATP synthesis
Atsuki Nakano, Jun-ichi Kishikawa, Nishida Yui, Kyosuke Sugawara, […] , and Ken Yokoyama
Science Advances Published:17 Oct 2025
DOI:https://doi.org/10.1126/sciadv.adx8771
Abstract
ATP synthases are rotary molecular machines that use the proton motive force to rotate the central rotor complex relative to the surrounding stator apparatus, thereby coupling the ATP synthesis. We reconstituted the V/A-ATPase into liposomes and performed structural analysis using cryo-EM under conditions where the proton motive force was applied in the presence of ADP and Pi. ATP molecules were bound at two of the three catalytic sites of V/A-ATPase, confirming that the structure represents a state adopted during ATP synthesis. In this structure, the catalytic site closes upon binding of ADP and Pi through an induced fit mechanism. Multiple structures were obtained where the membrane-embedded rotor ring was in a different position relative to the stator. By comparing these structures, we found that torsion occurs in both the central rotor and the peripheral stator during 31° rotation of rotor ring. These structural snapshots of V/A-ATPase provide crucial insights into the mechanism of rotary catalysis of ATP synthesis.
