2025-02-12 マックス・プランク研究所
<関連情報>
- https://www.mpg.de/24166261/0212-pfor-mechanism-for-treating-muscle-wasting-discovered-149770-x
- https://www.nature.com/articles/s41586-024-08539-x
デュシェンヌ型筋ジストロフィーにおけるユトロフィンの転写適応によるアップレギュレーション Transcriptional adaptation upregulates utrophin in Duchenne muscular dystrophy
Lara Falcucci,Christopher M. Dooley,Douglas Adamoski,Thomas Juan,Justin Martinez,Angelina M. Georgieva,Kamel Mamchaoui,Cansu Cirzi & Didier Y. R. Stainier
Nature Published:12 February 2025
DOI:https://doi.org/10.1038/s41586-024-08539-x
Abstract
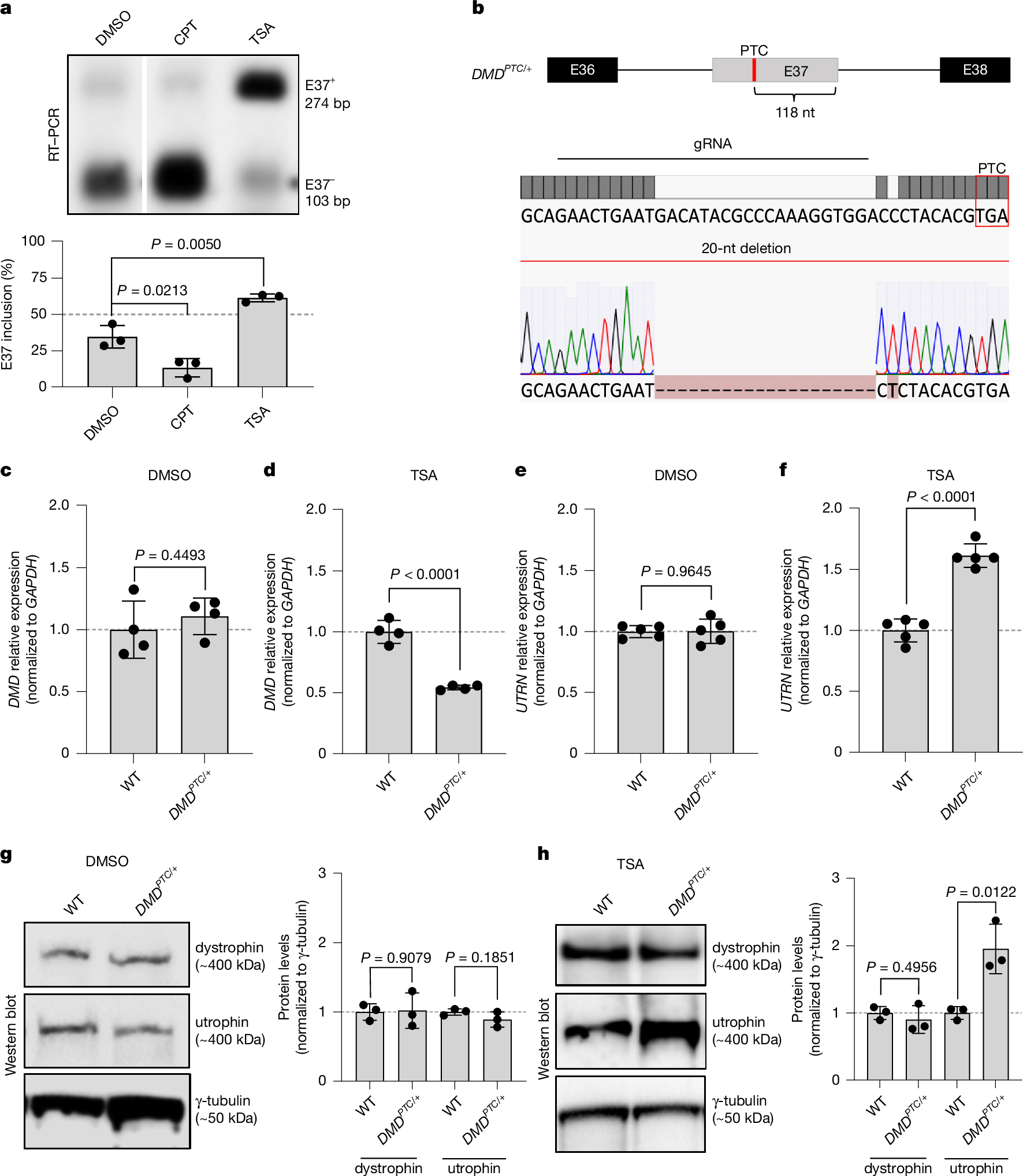
Duchenne muscular dystrophy (DMD) is a muscle-degenerating disease caused by mutations in the DMD gene, which encodes the dystrophin protein1,2. Utrophin (UTRN), the genetic and functional paralogue of DMD, is upregulated in some DMD patients3,4,5. To further investigate this UTRN upregulation, we first developed an inducible messenger RNA (mRNA) degradation system for DMD by introducing a premature termination codon (PTC) in one of its alternatively spliced exons. Inclusion of the PTC-containing exon triggers DMD mutant mRNA decay and UTRN upregulation. Notably, blocking nonsense-mediated mRNA decay results in the reversal of UTRN upregulation, whereas overexpressing DMD does not. Furthermore, overexpressing DMDPTC minigenes in wild-type cells causes UTRN upregulation, as does a wild-type DMD minigene containing a self-cleaving ribozyme. To place these findings in a therapeutic context, we used splice-switching antisense oligonucleotides (ASOs) to induce the skipping of out-of-frame exons of DMD, aiming to introduce PTCs. We found that these ASOs cause UTRN upregulation. In addition, when using an ASO to restore the DMD reading frame in myotubes derived from a DMDΔE52 patient, an actual DMD treatment, UTRN upregulation was reduced. Altogether, these results indicate that an mRNA decay-based mechanism called transcriptional adaptation6,7,8 plays a key role in UTRN upregulation in DMDPTC patients, and they highlight an unexplored therapeutic application of ASOs, as well as ribozymes, in inducing genetic compensation via transcriptional adaptation.


