2025-06-17 ロイヤルメルボルン工科大学(RMIT)
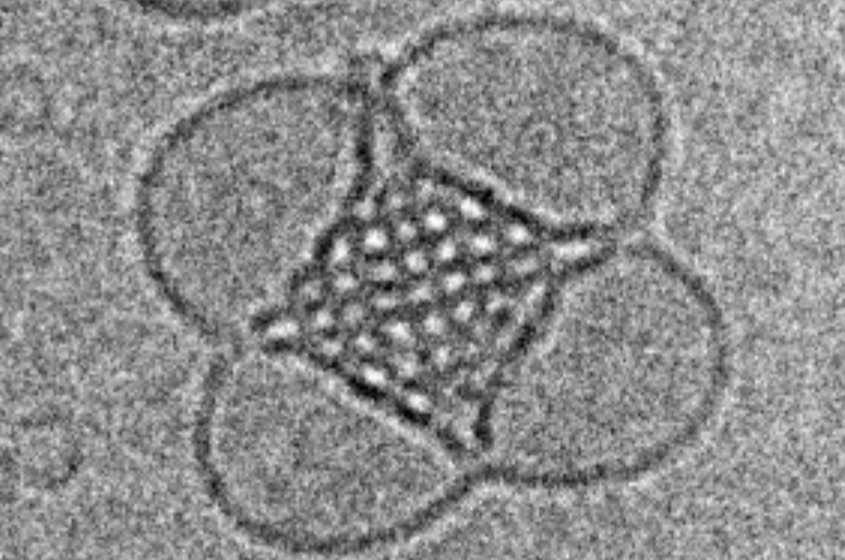
A cubosome nanoparticle magnified more than 30,000 times, showing its lipids self-assembled into a cubic structure.
<関連情報>
- https://www.rmit.edu.au/news/all-news/2025/jun/cubosomes
- https://onlinelibrary.wiley.com/doi/10.1002/smll.202500903
脂質ナノ粒子の内部ナノ構造が多様な細胞内取り込み経路に影響を与える The Internal Nanostructure of Lipid Nanoparticles Influences Their Diverse Cellular Uptake Pathways
Sue Lyn Yap, Brendan Dyett, Alison J. Hobro, Han Nguyen, Nicholas I. Smith, Calum J. Drummond, Charlotte E. Conn, Nhiem Tran
Small Published: 20 May 2025
DOI:https://doi.org/10.1002/smll.202500903
Abstract
Lipid nanoparticles have emerged as critical platforms for bioactive agent delivery, with their success in COVID-19 vaccines highlighting the urgent need to address gaps in understanding their biological interactions. Lyotropic liquid crystalline nanoparticles (LLCNPs) represent promising nanocarriers for bioactive agent delivery. In this study, it is revealed for the first time how internal nanostructures of LLCNPs – liposomes, cubosomes, hexosomes, and micellar cubosomes – influence their cellular uptake pathways. By isolating the effects of mesophase while maintaining consistent particle size, charge, and surface coating, it is demonstrated that non-lamellar LLCNPs, particularly cubosomes, significantly enhance cellular uptake via distinct endocytic and non-endocytic mechanisms. These nanoparticles predominantly utilize passive non-endocytic pathways, such as membrane fusion, bypassing endocytic recycling challenges faced by most nanomaterials, including lamellar liposomes. Among active endocytic pathways, macropinocytosis emerges as the dominant route for non-lamellar particles. The findings establish a direct link between LLCNP internal nanostructure and cellular internalization mechanisms, highlighting the critical role of mesophase design in optimizing nanocarrier performance. This knowledge enables the rational engineering of LLCNPs tailored to target specific uptake pathways, facilitating precision delivery for diverse therapeutic applications and addressing key barriers in intracellular drug transport.