2025-06-27 東北大学
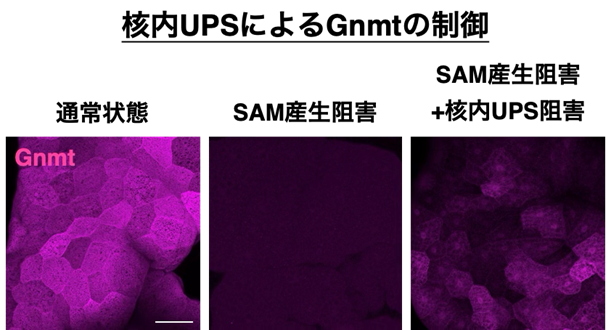
図1. 消費酵素Gnmtの減少が核内UPSによって制御される
通常状態では、Gnmtは主に細胞質に存在し、SAMを消費している。一方、SAMが産生されない状況ではGnmtは減少し、SAMの消費が抑制される。
このGnmtの減少は、核内のユビキチン·プロテアソームシステム(UPS)によって制御されており、核内UPSを阻害するとGnmtの減少は抑制され、核内にGnmtが蓄積する。
<関連情報>
- https://www.tohoku.ac.jp/japanese/2025/06/press20250627-02-SAM.html
- https://www.tohoku.ac.jp/japanese/newimg/pressimg/tohokuuniv-press20250627_02web_SAM.pdf
- https://www.pnas.org/doi/10.1073/pnas.2417821122
S-アデノシルメチオニン代謝の緩衝化は、核ユビキチン・プロテアソーム系を介したグリシンN-メチルトランスフェラーゼの減少によって制御される S-adenosylmethionine metabolism buffering is regulated by a decrease in glycine N-methyltransferase via the nuclear ubiquitin–proteasome system
Soshiro Kashio and Masayuki Miura
Proceedings of the National Academy of Sciences Published:June 24, 2025
DOI:https://doi.org/10.1073/pnas.2417821122
Significance
S-adenosylmethionine (SAM) metabolism is crucial for diverse functions, mediated through methylation. Although the feedback regulation of SAM production has been explored extensively, our understanding of the mechanism behind SAM consumption remains incomplete. Constant levels of SAM have been observed in Drosophila fat bodies even under conditions of SAM shortage, including nutrient deficiency and inhibition of SAM synthesis. SAM levels are controlled by the reduction in glycine N-methyltransferase (Gnmt), a cytosolic SAM-consuming enzyme, via the nuclear ubiquitin–proteasome system under conditions of SAM shortage. Additionally, inhibition of Gnmt level reduction by suppression of the nuclear UPS causes starvation tolerance. Considering that SAM accumulation promotes energy expenditure in vivo, the starvation-dependent mechanism of Gnmt reduction is important for energy homeostasis.
Abstract
Metabolic homeostasis is essential for survival; however, many studies have focused on the fluctuations of these factors. Furthermore, while metabolic homeostasis depends on the balance between the production and consumption of metabolites, there have been limited investigations into the mechanisms regulating their consumption. S-adenosylmethionine (SAM) metabolism has diverse functions, including methylation, polyamine biosynthesis, and transsulfuration, making its regulation and control crucial. Recent studies have revealed the feedback regulation of SAM production; however, the mechanisms governing its consumption are still poorly understood. In this study, we focused on the stability of SAM levels in the fat body (FB) of Drosophila, which serves as a functional equivalent of the mammalian liver and adipose tissue, under conditions of SAM shortage, including nutrient deprivation. We found that glycine N-methyltransferase (Gnmt), a major SAM-consuming methyltransferase in the FB, decreased via the nuclear ubiquitin–proteasome system (UPS), along with the inhibition of SAM synthesis and starvation. The inhibition of Gnmt level reduction by suppression of the nuclear UPS causes starvation tolerance. Thus, the regulation of Gnmt levels through nuclear UPS-mediated reduction helps maintain SAM levels under SAM shortage conditions.

