2025-08-20 中国科学院(CAS)
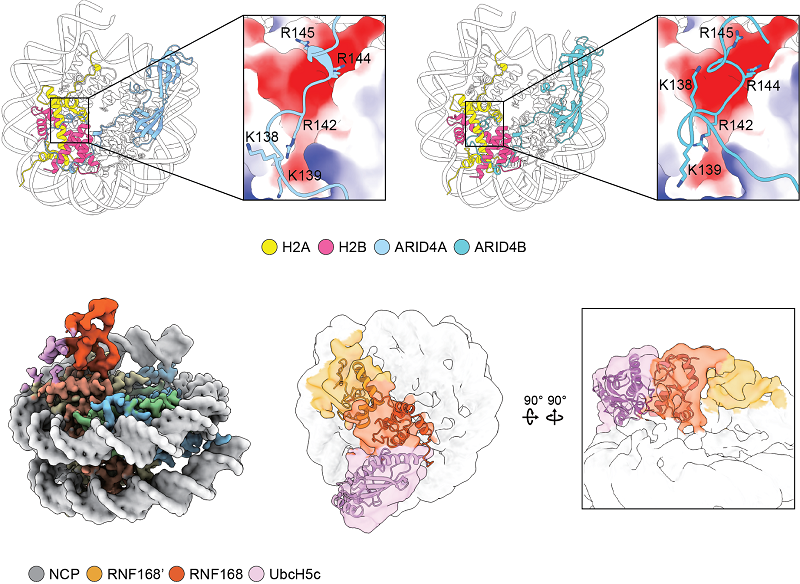
ARID4A/4B Recognition of the Nucleosome and RNF168 Dimerization Enhance Nucleosome Interactions (Image by ZHOU Zheng’s group)
<関連情報>
- https://english.cas.cn/newsroom/research_news/life/202508/t20250825_1051338.shtml
- https://academic.oup.com/nar/article/53/14/gkaf735/8223175?login=false
AlphaFoldによるヌクレオソーム結合タンパク質の構造解析 AlphaFold-guided structural analyses of nucleosome binding proteins
Xin Yang , Haoqiang Zhu , Liuxin Shi , Tingrui Song , Weibin Gong , Shunmin He , Shan Shan , Chunfu Xu , Zheng Zhou
Nucleic Acids Research Published:06 August 2025
DOI:https://doi.org/10.1093/nar/gkaf735
Abstract
The nucleosome, as the fundamental unit of chromatin, interacts with a diverse range of proteins, crucially regulating gene expression. In this study, we introduce an AlphaFold-based algorithm designed to analyze nucleosome-binding proteins from a dataset of over 7600 human nuclear proteins. Using proteins that interact with the nucleosome acidic patch as a benchmark, our screening achieves a successful prediction rate of 77% (23 out of 30 proteins). This predictive approach has led to the identification of ARID4A and ARID4B as novel nucleosome-binding proteins. Additionally, this analytical method was used to study RING-family ubiquitin E3 ligase RNF168, demonstrating that RNF168 dimerization enhances its binding to the nucleosome, a finding confirmed by cryogenic-electron microscopy structural analysis. Our findings offer a rapid and effective method for the discovery and characterization of nucleosome-binding proteins and emphasize the significant role of ubiquitin E3 ligase dimerization in epigenetic regulation.


