2025-10-08 カリフォルニア大学リバーサイド校(UCR)
<関連情報>
- https://news.ucr.edu/articles/2025/10/08/new-hope-ms
- https://www.nature.com/articles/s41598-025-20254-9
良好な薬物動態を有するクロロインダゾール系エストロゲン受容体βリガンドは、機能的再髄鞘形成と視力回復を促進する Chloroindazole based estrogen receptor β ligands with favorable pharmacokinetics promote functional remyelination and visual recovery
Micah Feri,Sung Hoon Kim,Flavio D. Cardenas,Alyssa M. Anderson,Brandon T. Poole,Devang Deshpande,Shane Desfor,Kelley C. Atkinson,Stephanie R. Peterson,Kendall W. Nettles,Jerome C. Nwachukwu,Moyinoluwa T. Ajayi,Fernando Beltran,David E. Martin,Julio Tapia,Carol D. Curtis,Martin I. Garcia-Castro,Benita S. Katzenellenbogen,John A. Katzenellenbogen & Seema K. Tiwari-Woodruff
Scientific Reports Published:08 October 2025
DOI:https://doi.org/10.1038/s41598-025-20254-9
Abstract
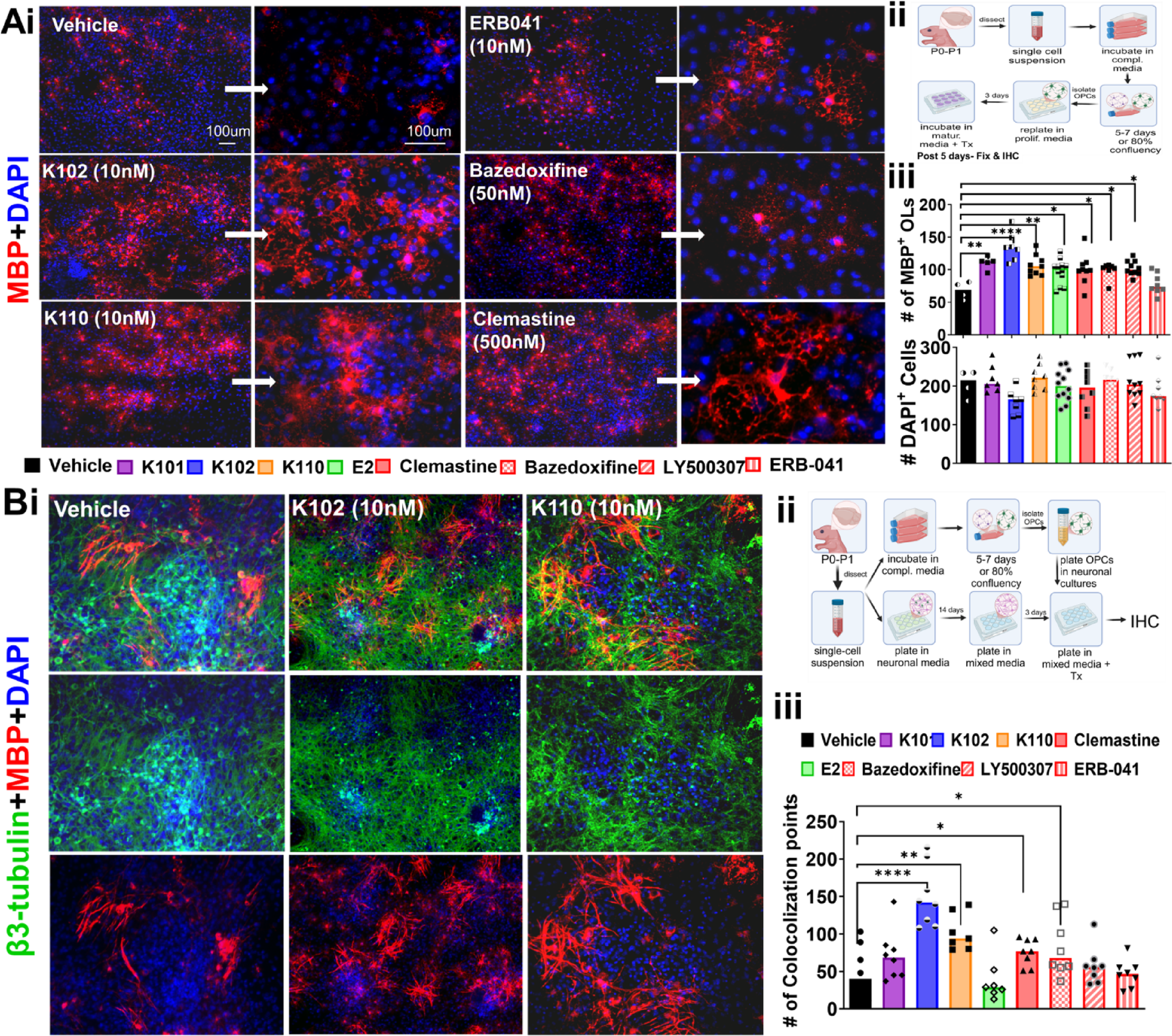
Multiple sclerosis (MS) is a chronic autoimmune, demyelinating, and neurodegenerative disease that results in motor, visual, and cognitive deficits. While existing treatments can slow disease progression, they rarely restore lost neurological function or significantly enhance quality of life. Estrogen receptor β (ERβ) has emerged as a promising therapeutic target due to its ability to activate non-classical signaling pathways involved in neuroprotection, immune modulation, and remyelination. In this study, two chloroindazole-based ERβ-selective ligands, K102 and K110, were identified for their favorable pharmacokinetic profiles and performance in preclinical absorption, distribution, metabolism, and elimination (ADME) screening. These compounds demonstrated biological activity by promoting oligodendrocyte (OL) differentiation in both primary mouse and human OL cultures. In vivo, they enhanced axonal remyelination and improved functional electrophysiological outcomes in two mouse models of MS: experimental autoimmune encephalomyelitis (EAE) and cuprizone diet-induced demyelination. Additionally, K102 and K110 modulated immune responses, supporting OL survival and contributing to motor and visual recovery in EAE mice. These findings provide compelling preclinical evidence for advancing K102 and K110 to clinical development. By simultaneously addressing neurodegeneration and inflammation through ERβ-mediated signaling, these compounds offer a novel and potentially transformative approach to MS therapy.