2025-10-10 マックス・プランク研究所
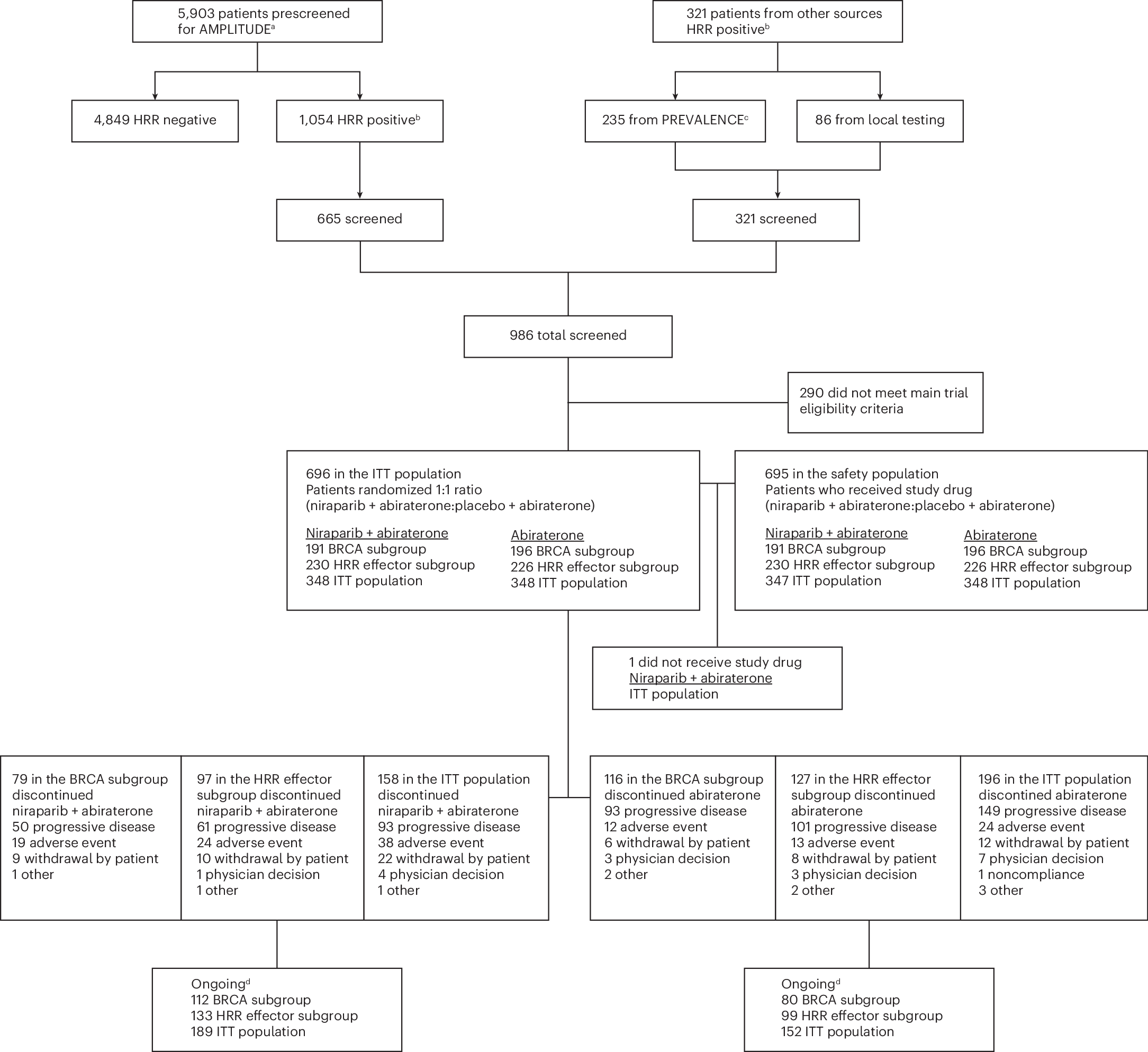
 Severing of actin filament (from grey to pink) in a multi-step process by the protein trio coronin (first purple, then blue), cofilin (green) and AIP1 (orange).
Severing of actin filament (from grey to pink) in a multi-step process by the protein trio coronin (first purple, then blue), cofilin (green) and AIP1 (orange).
© MPI f. Molecular Physiology
<関連情報>
- https://www.mpg.de/25534274/dancing-proteins-keep-cells-moving
- https://www.cell.com/cell/fulltext/S0092-8674(25)01084-0
コロニン、コフィリン、AIP1による急速なアクチンフィラメント分解の振り付け Choreography of rapid actin filament disassembly by coronin, cofilin, and AIP1
Wout Oosterheert ∙ Micaela Boiero Sanders ∙ Oliver Hofnagel ∙ Peter Bieling, ∙ Stefan Raunser
Cell Published:October 10, 2025
DOI:https://doi.org/10.1016/j.cell.2025.09.016
Highlights
- Cryo-EM reveals how coronin, cofilin, and AIP1 synergize to disassemble actin filaments
- Coronin induces phosphate release from F-actin to promote the rapid binding of cofilin
- Cofilin binds sequentially to both filament strands and sterically displaces coronin
- AIP1 severs cofilin-bound actin filaments through a “molecular squeezing” mechanism
Summary
Rapid remodeling of actin filament (F-actin) networks is essential for the movement and morphogenesis of eukaryotic cells. The conserved actin-binding proteins coronin, cofilin, and actin-interacting protein 1 (AIP1) act in synergy to promote rapid F-actin network disassembly, but the underlying mechanisms have remained elusive. Here, using cryo-electron microscopy (cryo-EM), we uncover the concerted molecular actions of coronin, cofilin, and AIP1 that lead to actin filament aging and severing. We find that the cooperative binding of coronin allosterically promotes inorganic phosphate release from F-actin and induces filament undertwisting, thereby priming the filament for cofilin binding. Cofilin then displaces coronin from the filament via a strand-restricted cooperative binding mechanism. The resulting cofilactin serves as a high-affinity platform for AIP1, which induces severing by acting as a clamp that disrupts inter-subunit filament contacts. In this “molecular squeezing” mechanism, AIP1 and not cofilin is responsible for filament severing. Our work redefines the role of key disassembly factors in actin dynamics.