2025-12-01 京都大学
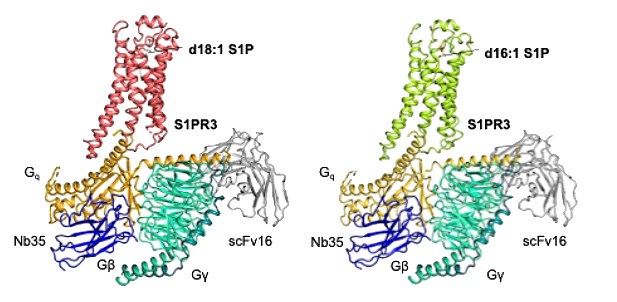
本研究で明らかにした2種類のS1PR3-Gq複合体の全体構造
<関連情報>
- https://www.kyoto-u.ac.jp/ja/research-news/2025-12-01-1
- https://www.kyoto-u.ac.jp/sites/default/files/2025-11/2511_PNAS_Hagiwara_relj-2-2webR-7226531a43cae61c8e994613ea97853b.pdf
- https://www.pnas.org/doi/10.1073/pnas.2507421122
ヒトスフィンゴシン-1-リン酸受容体3-Gq複合体によって明らかにされたGタンパク質サブタイプ選択性の構造的洞察 Structural insights into the G-protein subtype selectivity revealed by human sphingosine-1-phosphate receptor 3–Gq complexes
Momono Yamauchi, Dohyun Im, Shintaro Maeda, +9 , and Masatoshi Hagiwara
Proceedings of the National Academy of Sciences Published:November 18, 2025
DOI:https://doi.org/10.1073/pnas.2507421122
Significance
Sphingosine-1-phosphate (S1P) is an important bioactive lipid that regulates numerous essential immune functions through S1P receptors (S1PR1-5). Among them, S1PR3 plays critical roles in inflammatory responses by activating the Gαq signaling pathway, leading to leukocyte rolling. Here, we determined the structures of S1PR3-Gαq complexes bound to two endogenous agonists, providing the structural basis of Gαq signaling within the S1PR family. Although these agonists share similar chemical structures, we revealed that they activate the receptor through different mechanisms. We also identified key structural interactions that enable the same G-protein-coupled receptors (GPCR) to selectively engage different G proteins. These findings offer a unique perspective on G-protein selectivity in class A GPCRs that is distinct from the conventional views.
Abstract
Sphingosine-1-phosphate (S1P) is one of the most extensively studied bioactive lipids that transduces signals via the S1P receptor (S1PR) family (S1PR1-5), a class of G-protein-coupled receptors (GPCRs), to regulate immune cell migration, vascular permeability, and pain modulation. However, the mechanism for achieving specificity in downstream signaling remains poorly understood. Here, we present cryogenic electron microscopic structures of the S1PR3-Gαq complex bound to endogenous agonists: d18:1 S1P or d16:1 S1P. Both agonists shared the same binding pocket and binding mode despite the different signaling intensities of the S1PR3-Gαq signal pathway. By comparing the structures of two agonist-bound complexes, combined with mutagenesis studies, we identified key amino acids, Phe1193.33 and Arg1363.50, that play crucial roles in differential agonist recognition and receptor activation. Furthermore, structural comparisons with previously determined S1PR3-Gαi complex or G-protein-free S1PR3 structures, along with mutagenesis analysis, revealed dynamic intracellular loop 2 conformations and specific amino acid interactions that contribute to G-protein selectivity. Notably, we identified amino acids at the 34.50 and 34.53 positions within ICL2 as critical for specific interactions with G proteins. These findings provide better understanding of the mechanism of GPCR activation and unique perspectives that can be applied to other class A GPCRs, leading to the possibility of optimized drug development.

