2026-01-16 パシフィック・ノースウェスト国立研究所(PNNL)
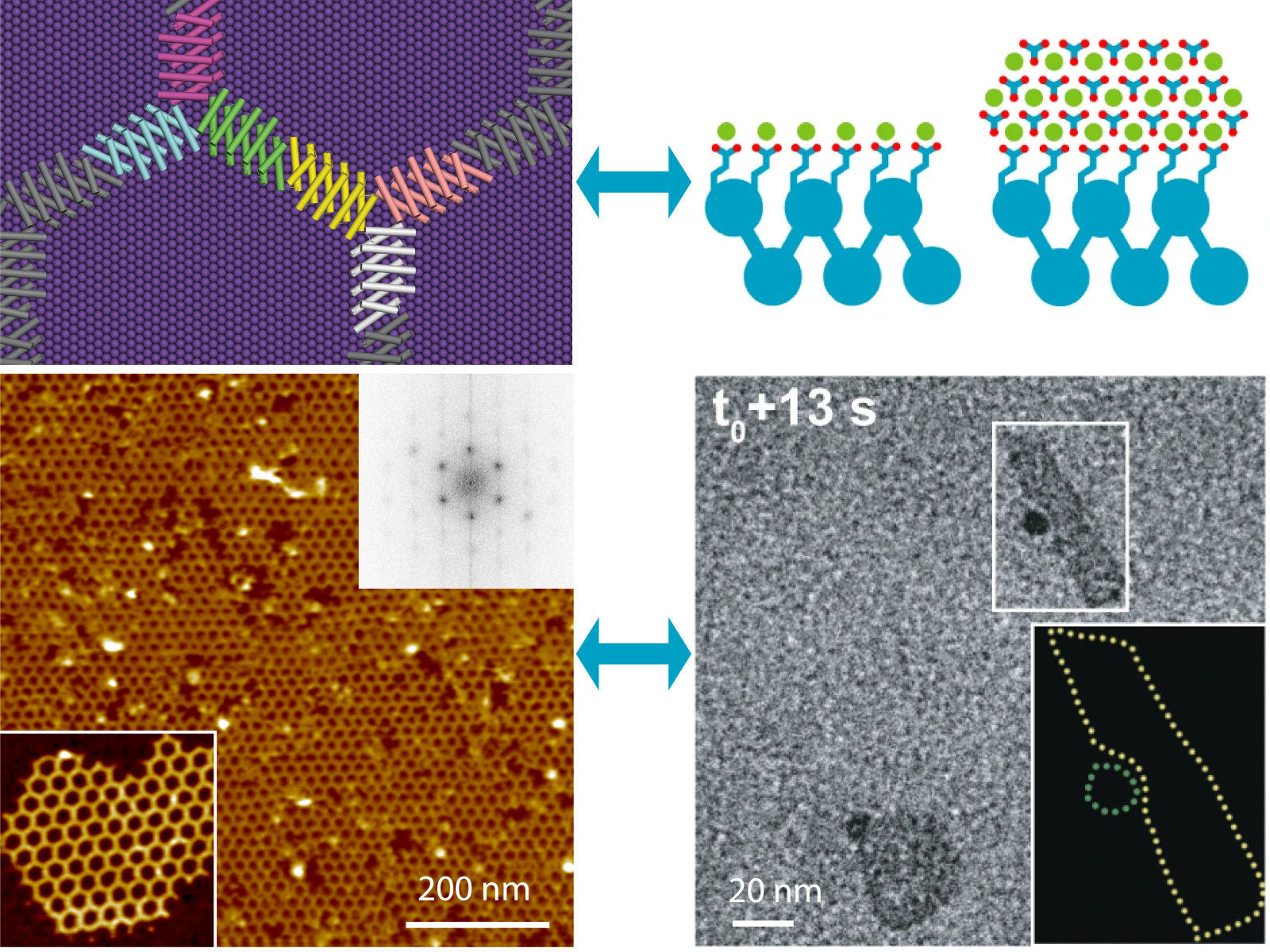
Complex material structures can form through templated crystal growth or direct protein self-assembly across a range of natural and designed proteins.(Figure by Shuai Zhang | Pacific Northwest National Laboratory; reproduced from s41586-019-1361-6 and s41467-023-43608-1)
<関連情報>
- https://www.pnnl.gov/publications/designing-protein-material-interfaces
- https://link.springer.com/article/10.1557/s43577-025-01008-4
タンパク質と物質の界面の設計 Designing protein–material interfaces
Shuai Zhang,Harley Pyles,David Baker & James J. De Yoreo
MRS Bulletin Published:11 December 2025
DOI:https://doi.org/10.1557/s43577-025-01008-4
Abstract
This article addresses recent advances in using de novo protein design to create coherent interfaces between proteins and inorganic materials, either through protein self-assembly on crystal lattices or through directed nucleation and growth of crystals by protein scaffolds. Inspired by natural protein–crystal interfaces, we focus on designed helical repeat proteins that present a repeating pattern of charged amino acid residues that epitaxially match a target inorganic crystal lattice. We describe the use of in situ imaging and spectroscopic methods to investigate both the assembly of these proteins and their ability to direct crystal nucleation and growth. The findings reveal the importance of surface charge, facet-specific binding, solvent organization, and, more generally, the balance of protein–substrate–solvent interactions in determining how organized protein–materials interfaces emerge. The results highlight the vast potential of protein design in materials science and inform our understanding of the mechanisms by which interactions between biomolecules and inorganic surfaces lead to unique materials and morphologies.