2026-01-30 千葉大学
<関連情報>
- https://www.chiba-u.ac.jp/news/research-collab/post_630.html
- https://www.chiba-u.ac.jp/news/files/pdf/0130_bunshi.pdf
- https://www.pnas.org/doi/10.1073/pnas.2508686123
キラルミオシン誘導アクチンダイナミクスの解明:単一フィラメント挙動から集団構造へ Elucidating chiral myosin–induced actin dynamics: From single-filament behavior to collective structures
Takeshi Haraguchi, Kohei Yoshimura, Yasuhiro Inoue, +6 , and Kohji Ito
Proceedings of the National Academy of Sciences Published:January 28, 2026
DOI:https://doi.org/10.1073/pnas.2508686123
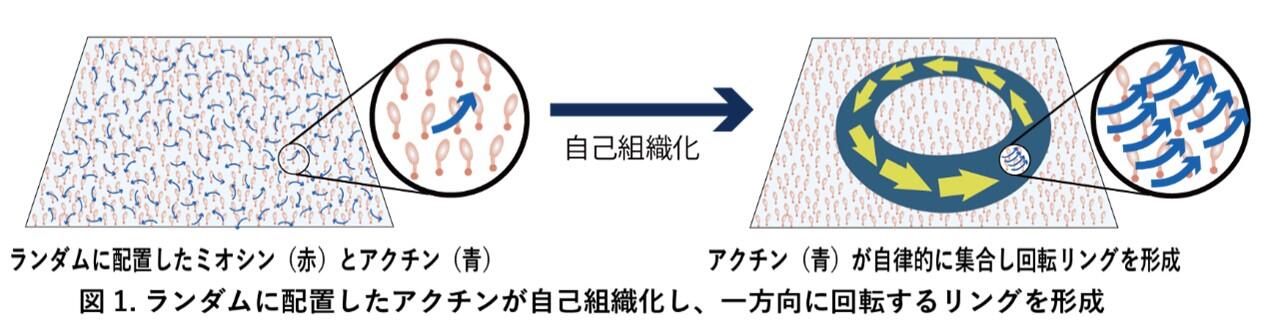
Significance
Myosins are motor proteins that move along actin filaments and underpin various intracellular functions. Recently, some myosins have been found to drive actin filaments along chiral curved paths, but the mechanisms and significance of this behavior remain largely unexplored. Here, we investigated this activity and found that it not only drives chiral curved motion of single actin filaments but also organizes them into stable, unidirectionally rotating ring structures through collective motion. These rings, termed actin chiral rings (ACRs), spontaneously emerge at high actin concentrations. Our findings uncover a organizing principle of actin self-assembly driven by myosin with chiral activity and provide a framework for understanding how cytoskeletal chirality is established.
Abstract
The myosin superfamily encompasses over 70 classes, each with multiple subclasses, and exhibits substantial diversity in properties such as velocity, ATPase activity, duty ratio, and directionality. This functional diversity enables the specialized roles of each myosin in various organisms, organs, and cell types. Beyond these well-characterized parameters, a newly recognized property has recently come into focus: Certain myosins drive actin filaments along chiral curved trajectories. However, this newly identified property remains largely unexplored. Here, we investigated this chiral motion in vitro using Chara corallina myosin XI (CcXI), which drives fast clockwise (CW) movement of actin filaments. This chiral motion arises from asymmetric displacement at the filament’s leading tip, and its curvature depends on the myosin density. Surprisingly, at elevated actin concentrations, filaments exhibiting chiral curved motion undergo collective dynamics, spontaneously forming a ring-shaped structure—termed the actin chiral ring (ACR)—that exhibits persistent CW rotation. ACRs display remarkable stability, continuing to rotate at their formation site until ATP is depleted, while maintaining their structure even after rotation ceases. This stability has not been reported among reported collective motions of cytoskeletal proteins driven by various motors. Our findings demonstrate that myosins with chiral activity can autonomously organize actin filaments into stable, chiral structures through collective motion, providing insights into actin self-organization by unconventional myosins. This paradigm offers a mechanistic basis for how motor-driven molecular asymmetry can give rise to coherent structural chirality at the cellular scale—an essential step in the emergence of cell chirality and asymmetry during development.


