2023-05-10 ピッツバーグ大学
◆このシステムは、T細胞と異なるタンパク質を標的とする抗体を融合させることで機能するため、治療法は柔軟でほぼ無限の可能性を持っています。また、共有結合を利用することで、より少量の抗体で受容体を活性化できるため、標的とするがんタンパク質の種類を広げることができます。
◆また、SNAP-SynNotchシステムは超プログラマブルであり、がん、自己免疫疾患、臓器移植耐性の遺伝子を提供できる可能性があります。
<関連情報>
- https://news.engineering.pitt.edu/customizing-t-cell-based-immunotherapies-in-a-snap/
- https://www.nature.com/articles/s41467-023-37863-5
CARとsynNotch受容体の翻訳後共有結合によるプログラマブル抗原ターゲティングの実現 Post-translational covalent assembly of CAR and synNotch receptors for programmable antigen targeting
Elisa Ruffo,Adam A. Butchy,Yaniv Tivon,Victor So,Michael Kvorjak,Avani Parikh,Eric L. Adams,Natasa Miskov-Zivanov,Olivera J. Finn,Alexander Deiters & Jason Lohmueller
Nature Communications Published:09 May 2023
DOI:https://doi.org/10.1038/s41467-023-37863-5
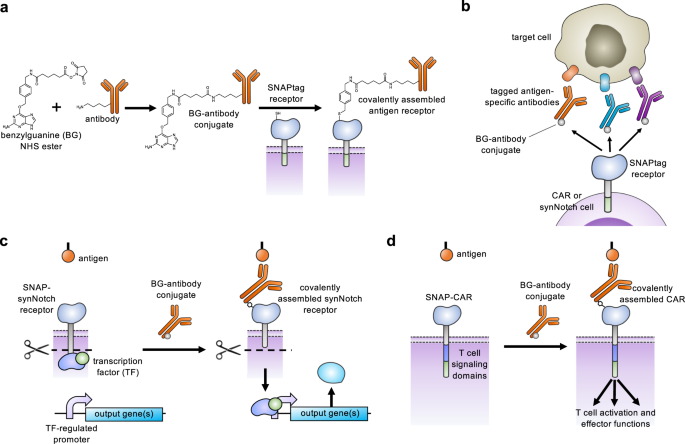
Abstract
Chimeric antigen receptors (CARs) and synthetic Notch (synNotch) receptors are engineered cell-surface receptors that sense a target antigen and respond by activating T cell receptor signaling or a customized gene program, respectively. Here, to expand the targeting capabilities of these receptors, we develop “universal” receptor systems for which receptor specificity can be directed post-translationally via covalent attachment of a co-administered antibody bearing a benzylguanine (BG) motif. A SNAPtag self-labeling enzyme is genetically fused to the receptor and reacts with BG-conjugated antibodies for covalent assembly, programming antigen recognition. We demonstrate that activation of SNAP-CAR and SNAP-synNotch receptors can be successfully targeted by clinically relevant BG-conjugated antibodies, including anti-tumor activity of SNAP-CAR T cells in vivo in a human tumor xenograft mouse model. Finally, we develop a mathematical model to better define the parameters affecting universal receptor signaling. SNAP receptors provide a powerful strategy to post-translationally reprogram the targeting specificity of engineered cells.