2025-07-22 東京大学
twip1による{110}双晶誘導を介したアラゴナイトの機械的強度向上機構
<関連情報>
- https://www.a.u-tokyo.ac.jp/topics/topics_20250722-1.html
- https://www.pnas.org/doi/10.1073/pnas.2503336122
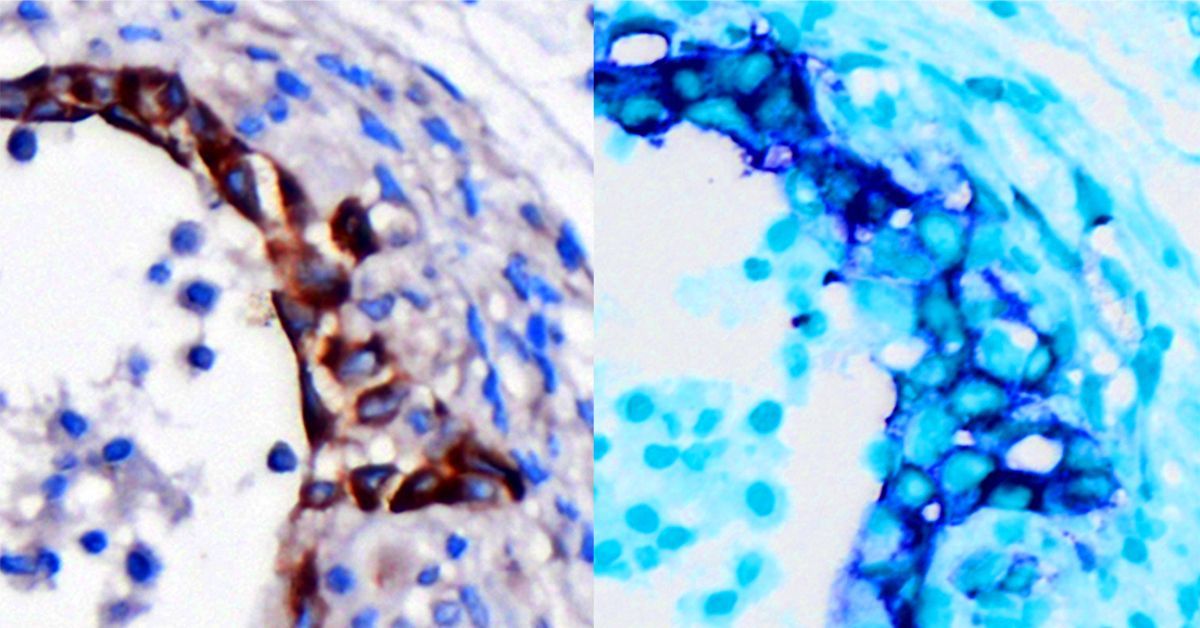
Glnリッチタンパク質twip1は、イシガイCellana rotaの殻における高密度{110}双子形成を促進する The Gln-rich protein twip1 promotes high-density {110} twins formation in the shell of the limpet Cellana rota
Sicheng Li, Xinlu Liu, Zehua Zheng, +8 , and Michio Suzuki,
Proceedings of the National Academy of Sciences Published:July 22, 2025
DOI:https://doi.org/10.1073/pnas.2503336122
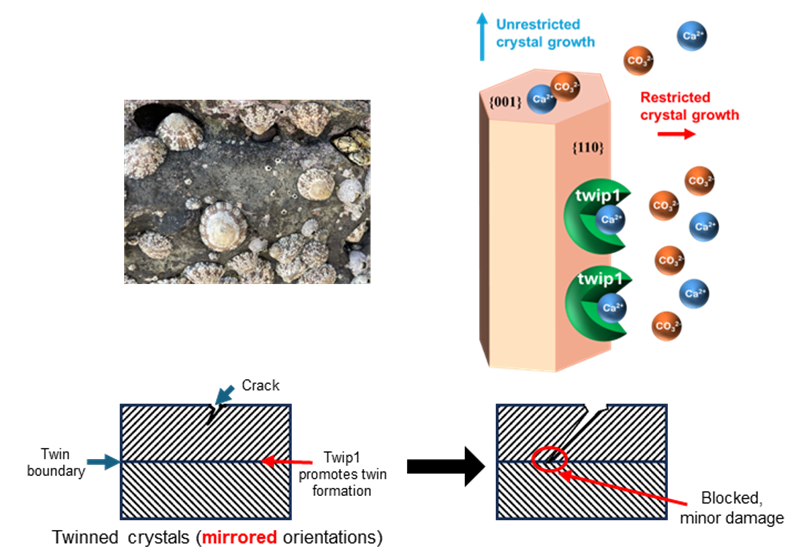
Significance
Biomineralization, the process by which living organisms form complex mineral structures, holds great potential for applications in materials science and bioengineering. Aragonite, a polymorph of calcium carbonate (CaCO3), is widely found in both biological and geological environments. One intriguing feature of aragonite is the formation of {110} twins, a crystal phenomenon that has been commonly observed but not well understood. It plays a critical role in enhancing toughness and elasticity. This study identifies a protein, twip1, which markedly promotes the density of {110} twins in aragonite crystals. By elucidating this biological mechanism, this research integrates biomolecular regulation into industrial material synthesis, providing a way for the development of bioinspired materials with tunable mechanical properties.
Abstract
Aragonite, a polymorph of calcium carbonate (CaCO3), exhibits enhanced mechanical properties due to crystal defects such as twins, which are particularly prevalent in biogenic aragonite and can inhibit crack propagation. Despite the importance, the molecular mechanisms responsible for the formation of aragonite {110} twins in marine organisms have remained poorly understood. Identifying a key inducer for {110} twins could help us understand how crystal defects are controlled in biomineralization. In this study, we explore the role of a protein, twip1, in inducing a high density of {110} twins within the aragonite layers of the limpet Cellana rota shell. Through a combination of layer-specific protein profiling and in vitro aragonite binding assays, twip1 is identified as a potential protein preferentially binding to the aragonite (110) plane. Gene expression analysis via quantitative PCR (qPCR) confirmed that twip1 is specifically expressed in the mantle tissue. To further investigate its function, recombinant twip1 (rtwip1) is produced using an Escherichia coli expression system. X-ray diffraction (XRD) and electron microscopy observations reveal that rtwip1 significantly increases {110} twin density during in vitro aragonite synthesis. Moreover, in vivo knockdown shows that reducing twip1 expression results in abnormal shell growth and {110} twin boundary reduction. Enhancing the formation of {110} twins in aragonite can improve its toughness and elasticity. This research highlights the crucial role of specific proteins in regulating crystal defect formation, offering a promising direction for future biomineralization studies and advanced material design.