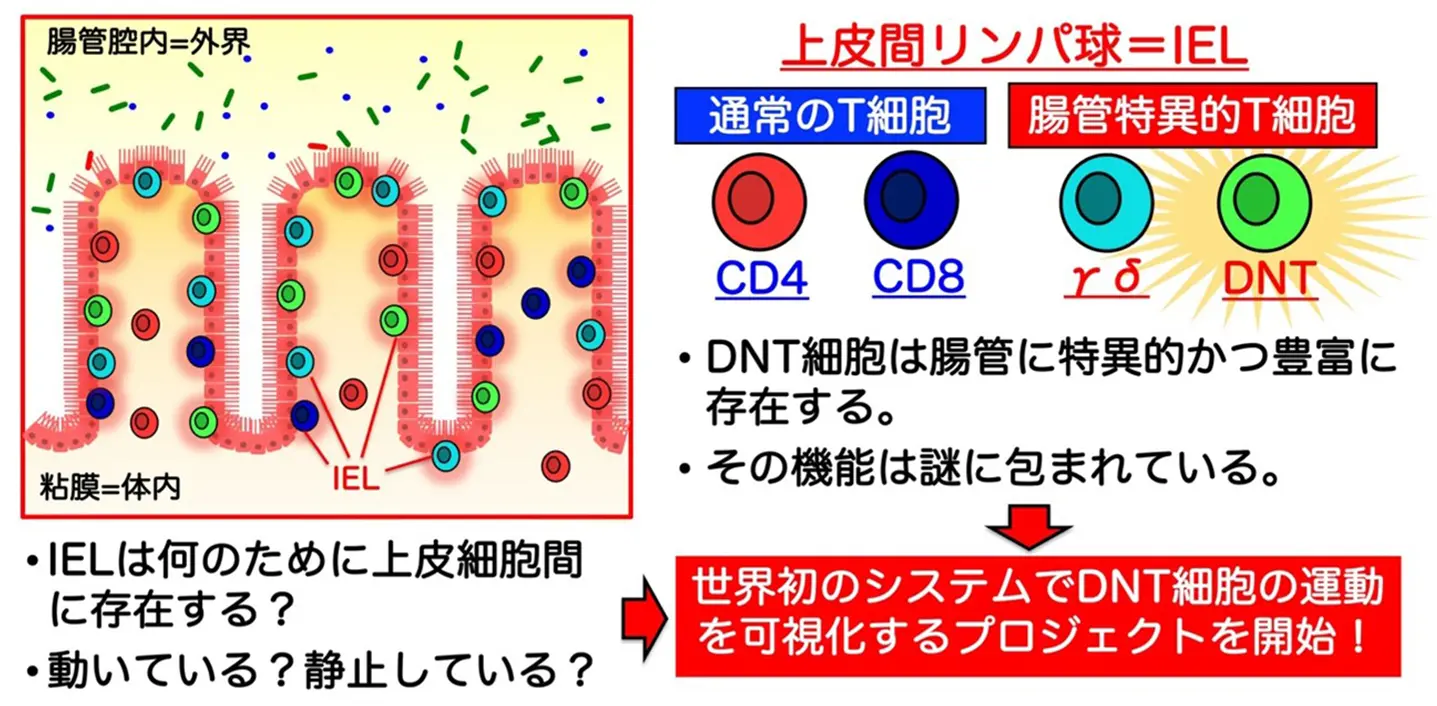
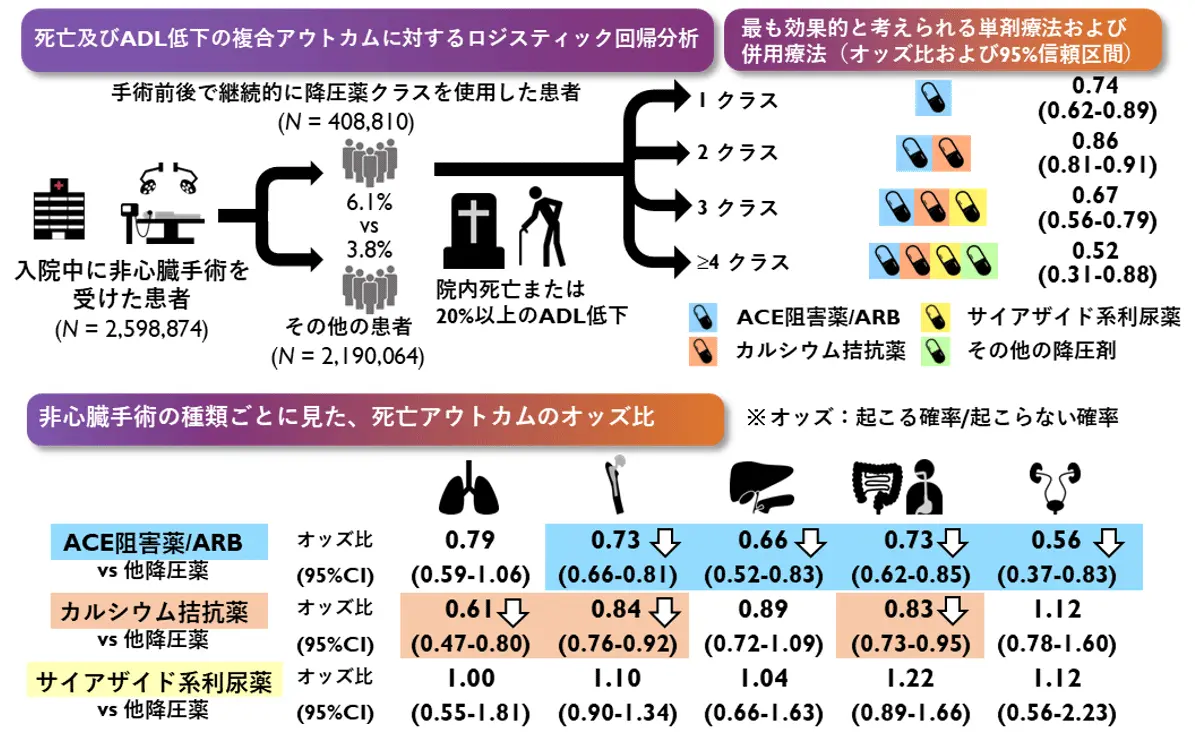
2025-08-29 東京科学大学
Web要約 の発言:
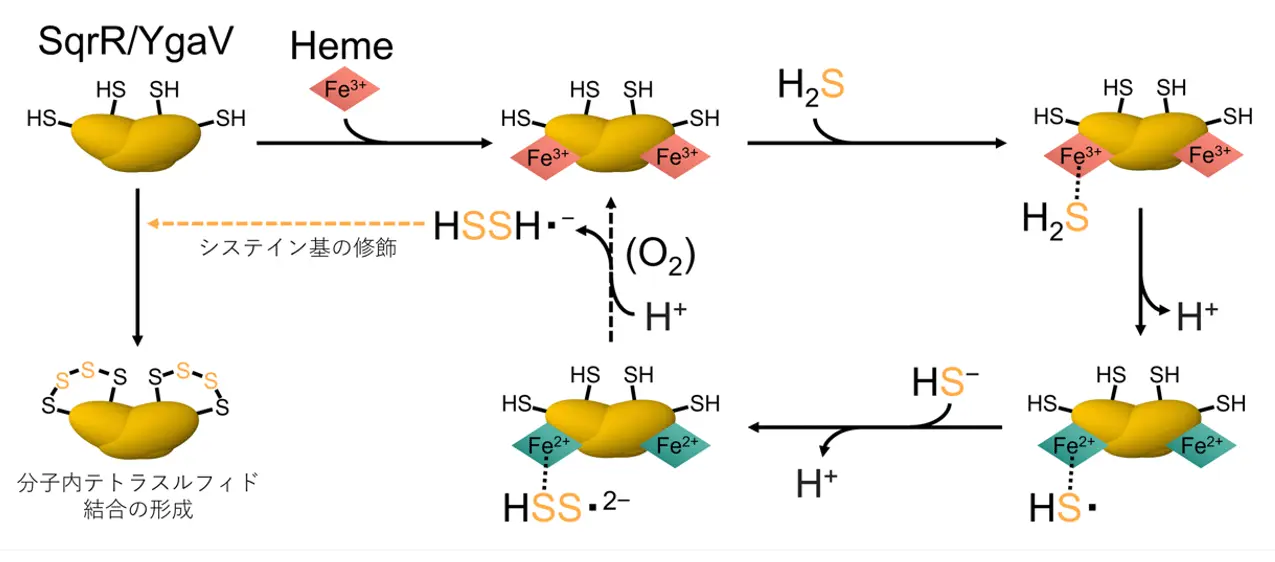
図1.ヘム鉄(Fe2+またはFe3+)と酸素(O2)に依存した硫化水素(H2S)による転写因子(SqrRあるいはYgaV)のシステイン残基のテトラスルフィド結合形成機構
<関連情報>
- https://www.isct.ac.jp/ja/news/g27kwusr4u7x
- https://www.isct.ac.jp/plugins/cms/component_download_file.php?type=2&pageId=&contentsId=1&contentsDataId=2149&prevId=&key=76b496dfe8a22e3119e8f160c844ebf0.pdf
- https://www.sciencedirect.com/science/article/pii/S2213231725003143
細菌転写因子SqrR/YgaVに結合したヘムは、酸素依存的に硫化水素を多硫化物へ変換し、遺伝子発現を制御する Heme bound to the bacterial transcription factor SqrR/YgaV catalyzes oxygen-dependent conversion of hydrogen sulfide to polysulfide for regulated gene expression
Ryoma Iwata, Shinji Masuda
Redox Biology Available online: 31 July 2025
DOI:https://doi.org/10.1016/j.redox.2025.103801
Highlights
- SqrR/YgaV specifically respond to polysulfides.
- Heme binding is required for SqrR/YgaV to respond to hydrogen sulfide.
- Heme-bound SqrR/YgaV catalyzes H2S conversion into polysulfides.
- Oxygen is required for heme-dependent synthesis of polysulfides.
Abstract
Hydrogen sulfide (H2S) and polysulfide are critical signaling molecules in bacteria, with distinct roles in regulating oxidative stress and redox balance. This study investigates the molecular mechanisms underlying the sulfur sensing and regulatory functions of two homologous transcription factors, SqrR from Rhodobacter capsulatus and YgaV from Escherichia coli. In vitro thiol-specific labeling and SDS–PAGE analyses demonstrate that apo-SqrR and apo-YgaV respond selectively and sensitively to polysulfides, rather than H2S itself, under both aerobic and anaerobic conditions. UV–visible spectroscopy demonstrated that the coordination state of the heme changes depending on the cysteine redox status: reduced cysteines support a six-coordinate heme, while oxidation to tetrasulfide crosslink leads to a five-coordinate state. Importantly, heme binding enhances cysteine oxidation by H2S under aerobic conditions, but not under anaerobic conditions, indicating that oxygen facilitates heme-mediated generation and utilization of polysulfides. In contrast, heme binding suppresses cysteine reactivity toward polysulfides under anaerobic conditions in both proteins, with this suppression modulated by the redox state of the heme iron. These findings suggest that heme binding regulates sulfur responsiveness by promoting cysteine oxidation by H2S under aerobic conditions and suppressing polysulfide reactivity under anaerobic conditions. This work reveals a context-dependent regulatory mechanism by which bacterial transcription factors integrate redox cues and sulfur metabolism, shedding light on their evolutionary adaptation to fluctuating oxygen and sulfur environments.