2025-11-06 東京大学
トリプトファン位置異性体による抗菌ペプチドグラミシジン A の性質改変
<関連情報>
- https://www.u-tokyo.ac.jp/focus/ja/press/z0111_00091.html
- https://www.u-tokyo.ac.jp/content/400273848.pdf
- https://pubs.acs.org/doi/10.1021/jacsau.5c00969
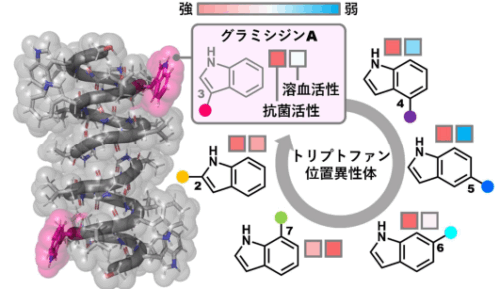
トリプトファンの位置異性体が物理化学的および生物学的特性に与える影響:グラミシジンA類似体を用いた事例研究 Impact of Tryptophan Positional Isomerism on Physicochemical and Biological Properties: A Case Study Using Gramicidin A Analogs
Takahiro Migita,Hiroaki Itoh,Hiroshi Hamamoto,and Masayuki Inoue
JACS Au Published: October 31, 2025
DOI:https://doi.org/10.1021/jacsau.5c00969
Abstract
Tryptophan (Trp) displays unique physicochemical properties due to its C3-substituted indole ring, containing a hydrophobic benzene ring and a hydrophilic N–H bond. Herein, we synthetically incorporated five Trp positional isomers with C2/4/5/6/7-substituted indoles in place of the Trp of residue 11 in gramicidin A, a 15-mer linear peptidic natural product. Gramicidin A conducts monovalent cations across the cell membrane and exhibits potent toxicity against both bacterial and mammalian cells. Our functional evaluation of the five analogs revealed that positional isomerism controlled the overall hydrophobicity and biological activities for the first time. Most importantly, we found that the hydrophobicity of the analogs correlated with the potency of mammalian cytotoxicity but not with the strength of the antibacterial activity, indicating that antibacterial and mammalian toxicities can be separated only by tuning the hydrophobicity. In addition, we designed and synthesized a triply mutated analog, in which the original valine, leucine, and Trp were replaced with less hydrophobic threonine, valine, and a C5-isomer, respectively. While the original antibacterial activity was maintained, the mammalian toxicity of the analog was more than 20-fold weaker. Consequently, these new findings offer a novel molecular editing approach to optimize the physicochemical and biological properties of Trp-containing bioactive peptides and proteins.