2025-11-21 ミュンヘン大学(LMU)
<関連情報>
- https://www.lmu.de/en/newsroom/news-overview/news/colliding-ribosomes-signal-cellular-stress.html
- https://www.nature.com/articles/s41586-025-09772-8
衝突したリボソームでのZAK活性化 ZAK activation at the collided ribosome
Vienna L. Huso,Shuangshuang Niu,Marco A. Catipovic,James A. Saba,Timo Denk,Eugene Park,Jingdong Cheng,Otto Berninghausen,Thomas Becker,Rachel Green & Roland Beckmann
Nature Published:19 November 2025
DOI:https://doi.org/10.1038/s41586-025-09772-8
Abstract
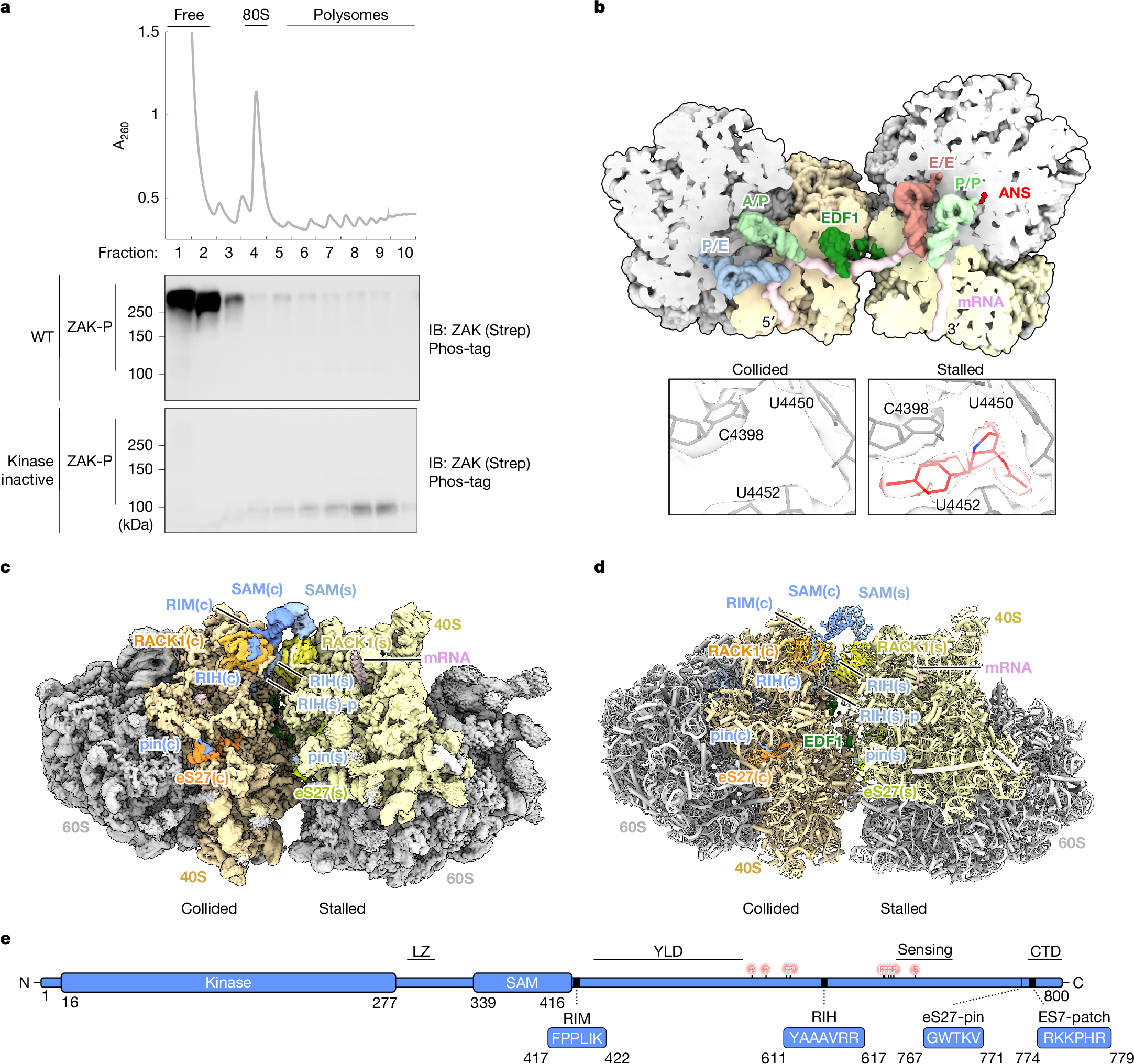
Ribosome collisions activate the ribotoxic stress response mediated by the MAP3K ZAK, which in turn regulates cell-fate consequences through downstream phosphorylation of the MAPKs p38 and JNK1. Despite the critical role of ZAK during cellular stress, a mechanistic and structural understanding of ZAK–ribosome interactions and how these lead to activation remain elusive. Here we combine biochemistry and cryo-electron microscopy to discover distinct ZAK–ribosome interactions required for constitutive recruitment and for activation. We find that upon induction of ribosome collisions, interactions between ZAK and the ribosomal protein RACK1 enable its activation by dimerization of its SAM domains at the collision interface. Furthermore, we discover how this process is negatively regulated by the ribosome-binding protein SERBP1 to prevent constitutive ZAK activation. Characterization of novel SAM variants as well as a known pathogenic variant of the SAM domain of ZAK supports a key role of the SAM domain in regulating kinase activity on and off the ribosome, with some mutants bypassing the ribosome requirement for ZAK activation. Collectively, our data provide a mechanistic blueprint of the kinase activity of ZAK at the collided ribosome interface.
