(Purdue University photo/Xing-Qi Huang)
<関連情報>
- https://www.purdue.edu/newsroom/releases/2022/Q1/new-and-improved-cherry-flavor-courtesy-of-the-petunia-flower.html
- https://www.nature.com/articles/s41467-022-28978-2?_ga=2.86533279.64289726.1647914682-828432578.1646956741
植物におけるペルオキシソームのヘテロ二量体酵素がベンズアルデヒド合成に関与すること A peroxisomal heterodimeric enzyme is involved in benzaldehyde synthesis in plants
Xing-Qi Huang,Renqiuguo Li,Jianxin Fu &Natalia Dudareva
Published:
Abstract
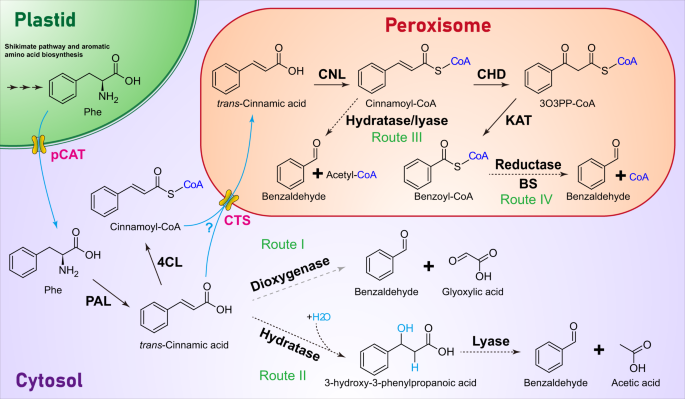
Benzaldehyde, the simplest aromatic aldehyde, is one of the most wide-spread volatiles that serves as a pollinator attractant, flavor, and antifungal compound. However, the enzyme responsible for its formation in plants remains unknown. Using a combination of in vivo stable isotope labeling, classical biochemical, proteomics and genetic approaches, we show that in petunia benzaldehyde is synthesized via the β-oxidative pathway in peroxisomes by a heterodimeric enzyme consisting of α and β subunits, which belong to the NAD(P)-binding Rossmann-fold superfamily. Both subunits are alone catalytically inactive but, when mixed in equal amounts, form an active enzyme, which exhibits strict substrate specificity towards benzoyl-CoA and uses NADPH as a cofactor. Alpha subunits can form functional heterodimers with phylogenetically distant β subunits, but not all β subunits partner with α subunits, at least in Arabidopsis. Analysis of spatial, developmental and rhythmic expression of genes encoding α and β subunits revealed that expression of the gene for the α subunit likely plays a key role in regulating benzaldehyde biosynthesis.