MINFLUX技術をより高い空間・時間精度にまで高めることで、生理的条件下でのタンパク質ダイナミクスを観察できるようになった Pushing the MINFLUX technique to higher spatial and temporal precision allows protein dynamics to be observed under physiological conditions
2023-03-09 マックス・プランク研究所
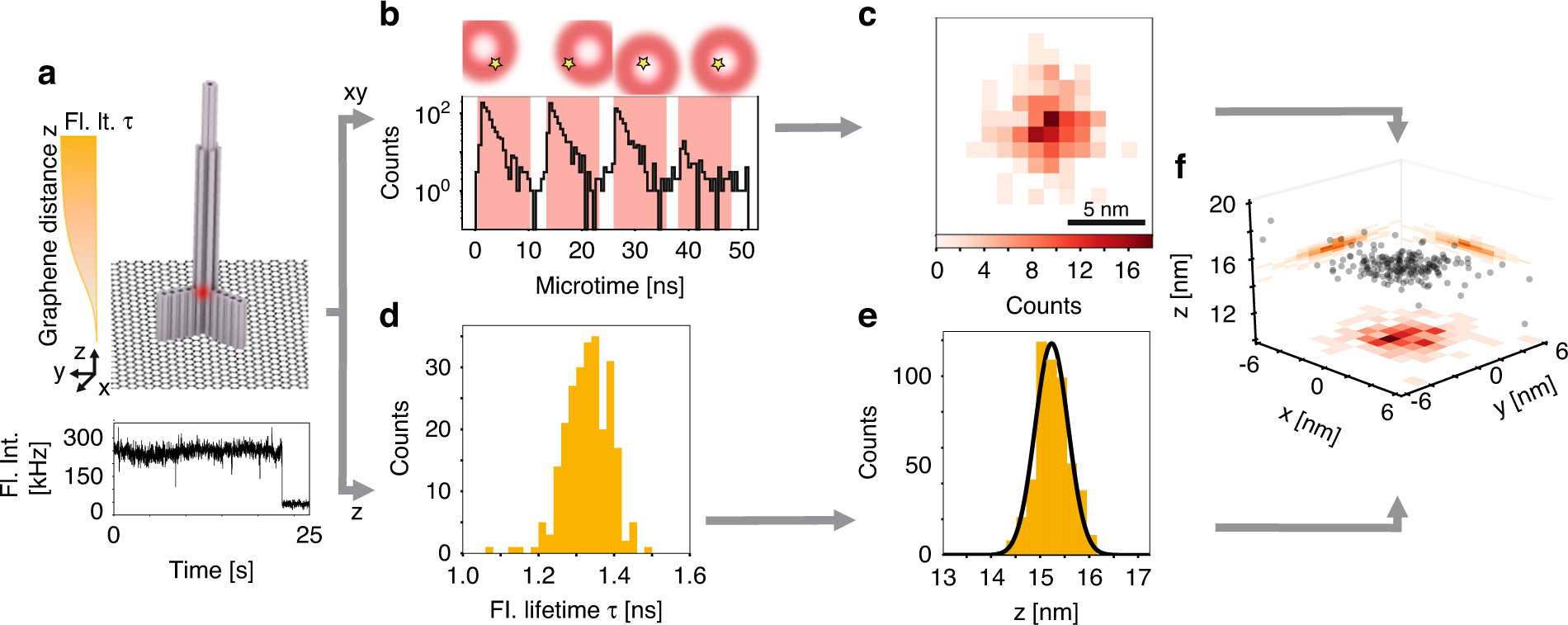
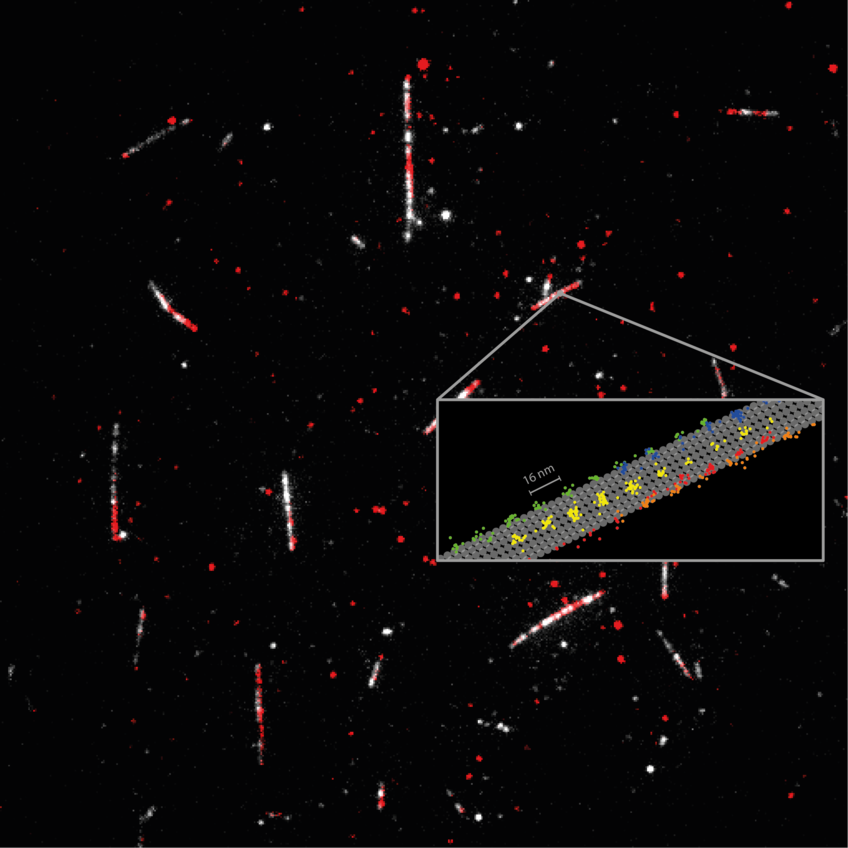 Detailed measurement of how the motor protein kinesin-1 (red) walks on microtubules (white). Tracking of the 2D movements of individual kinesin-1 dimers (color coded on the sketch) at physiological ATP concentrations revealed key details of how the protein walks in individual lanes. MINFLUX facilitated near protofilament tracking of the motor protein on the microtubule (sketched in grey).
Detailed measurement of how the motor protein kinesin-1 (red) walks on microtubules (white). Tracking of the 2D movements of individual kinesin-1 dimers (color coded on the sketch) at physiological ATP concentrations revealed key details of how the protein walks in individual lanes. MINFLUX facilitated near protofilament tracking of the motor protein on the microtubule (sketched in grey).
© MPI for Medical Research
超解像度顕微鏡(すなわちナノスコピー)を用いた蛍光顕微鏡は、この分野で欠かせない存在となっている。しかし、分子細胞生理学の理解を更に高次元にするには、より高い空間・時間分解能での観察が必要である。
ドイツのマックス・プランク医学研究所の研究者らは、最近導入された蛍光顕微鏡システムMINFLUXを使用して、単一分子レベルでタンパク質の構造変化を追跡することに成功した。MINFLUXは、わずか1nmのサイズを持つ標識に対してのみ必要であり、タンパク質の機能を妨げることなく、生体内におけるタンパク質ダイナミクスを研究するために必要な分解能と最小限の侵入性を提供できる。
今後、MINFLUXは、タンパク質ダイナミクスの新たな研究分野を開拓し、多くの病気のメカニズムの理解に役立つ可能性がある。
<関連情報>
- https://www.mpg.de/19988355/0309-mefo-fast-even-at-the-nanometer-level-153070-x
- https://www.science.org/doi/10.1126/science.ade2650
MINFLUXは、キネシン-1の歩行の妨げにならないように解剖している MINFLUX dissects the unimpeded walking of kinesin-1
Jan O. Wolff,Lukas Scheiderer,Tobias Engelhardt,Johann Engelhardt,Jessica Matthias,Stefan W. Hell
Science Published:9 Mar 2023
Zeroing in on motor proteins
The super-resolution microscopy technique MINFLUX enables localization of fluorophores using a minimal number of photons. Two studies now expand on the development and implementation of MINFLUX to track motor protein dynamics in vitro and in cells (see the Perspective by Fei and Zhou). Wolff et al. refined the precision of MINFLUX such that single-fluorophore tracking with nanometer precision was possible with only tens of photons. They tracked the movement of kinesin-1 on microtubules and were able to see individual 4-nanometer substeps and rotation of the protein during stepping in their analysis. Deguchi et al. applied MINFLUX with a labeling and tracking approach called motor-PAINT to monitor stepping of motor proteins on microtubules in living and fixed cells in both two and three dimensions. —MAF
Abstract
We introduce an interferometric MINFLUX microscope that records protein movements with up to 1.7 nanometer per millisecond spatiotemporal precision. Such precision has previously required attaching disproportionately large beads to the protein, but MINFLUX requires the detection of only about 20 photons from an approximately 1-nanometer-sized fluorophore. Therefore, we were able to study the stepping of the motor protein kinesin-1 on microtubules at up to physiological adenosine-5′-triphosphate (ATP) concentrations. We uncovered rotations of the stalk and the heads of load-free kinesin during stepping and showed that ATP is taken up with a single head bound to the microtubule and that ATP hydrolysis occurs when both heads are bound. Our results show that MINFLUX quantifies (sub)millisecond conformational changes of proteins with minimal disturbance.