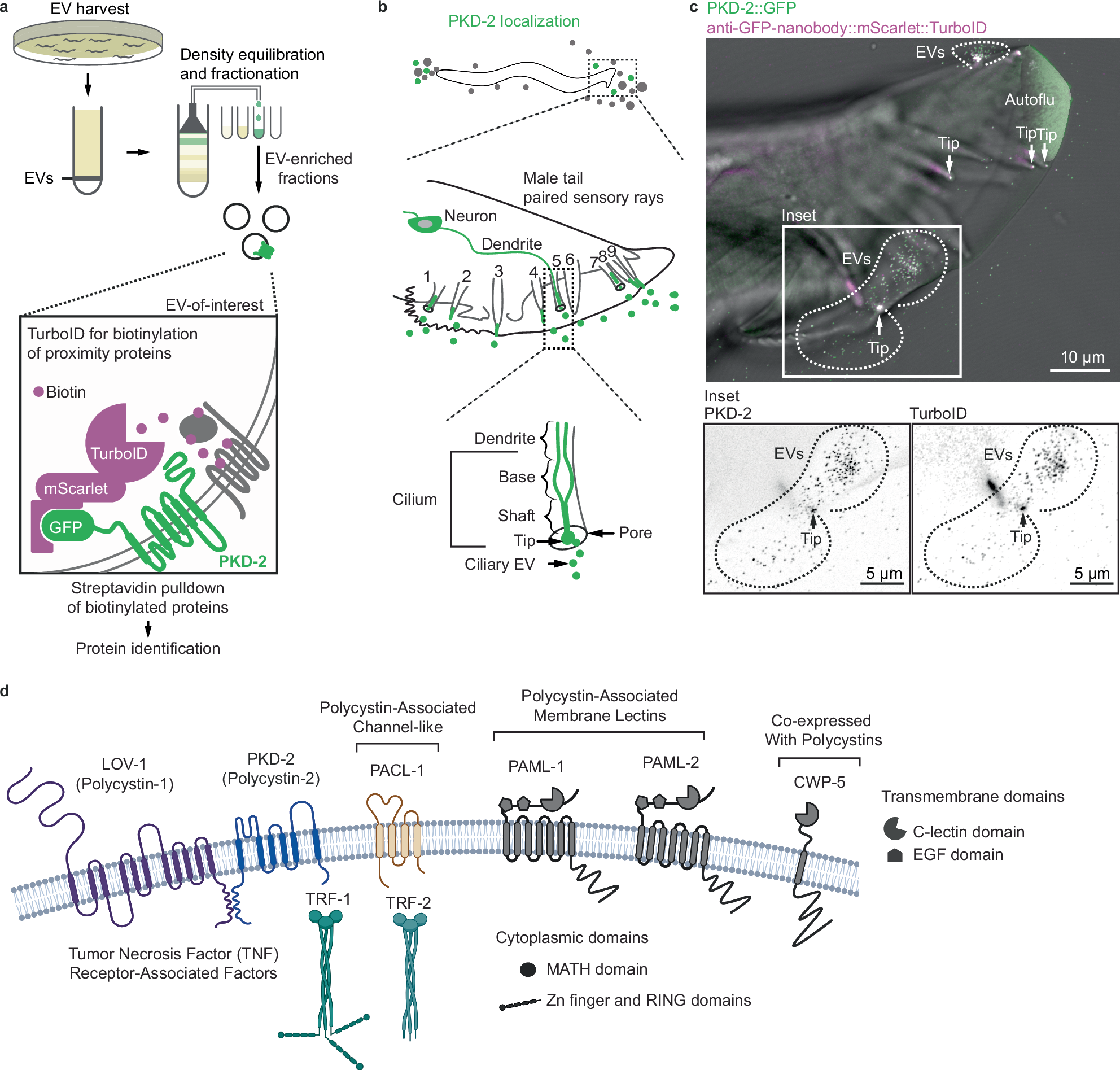
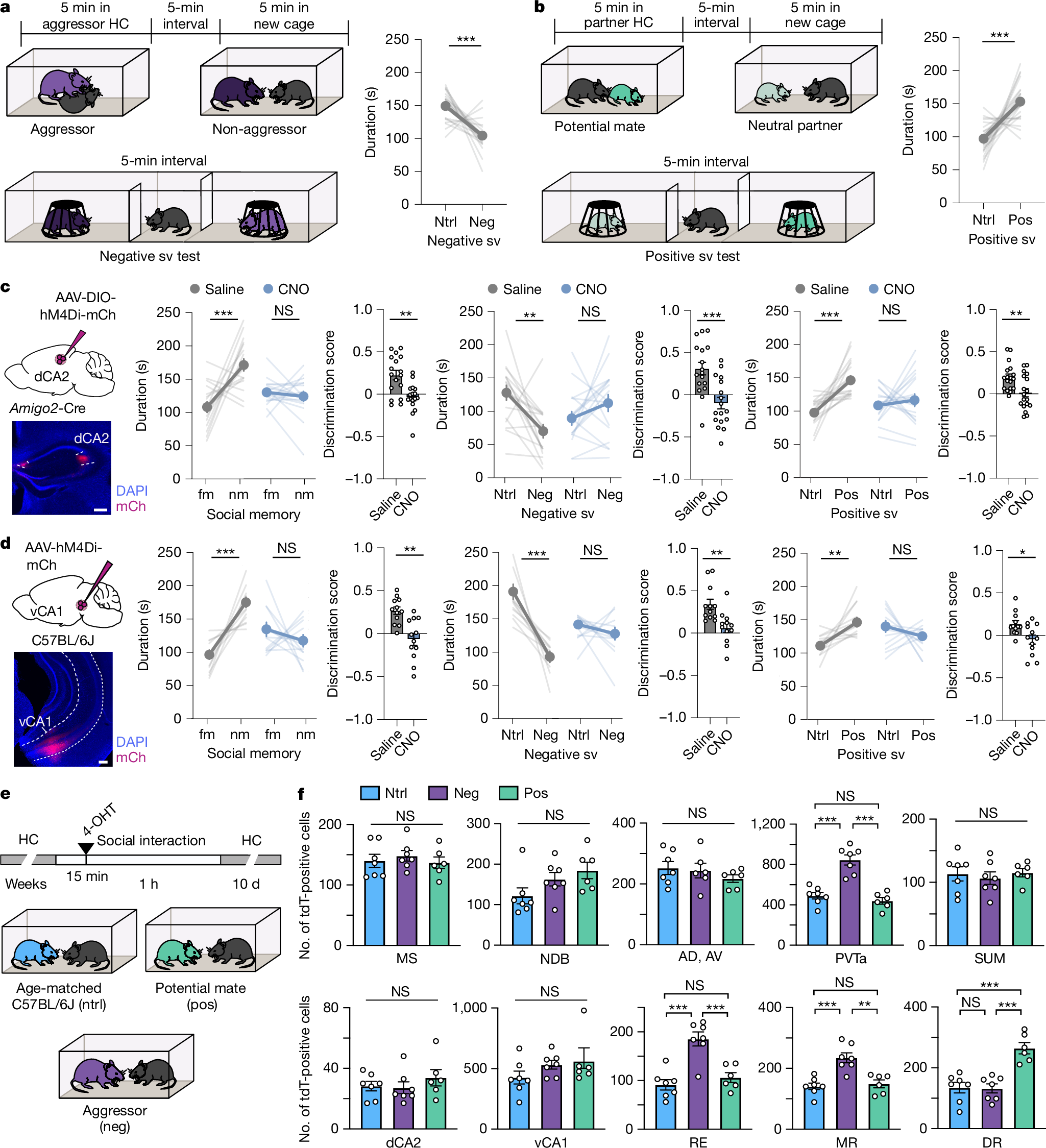
2025-04-30 マウントサイナイ医療システム (MSHS)
<関連情報>
- https://www.mountsinai.org/about/newsroom/2025/mount-sinai-researchers-discover-how-melanoma-may-spread-to-the-brain
- https://www.sciencedirect.com/science/article/pii/S109727652500303X?dgcid=coauthor
SWI/SNF PBAF複合体は抑制クロマチンにおけるRESTの占有を促進する The SWI/SNF PBAF complex facilitates REST occupancy at repressive chromatin
Elena Grossi, Christie B. Nguyen, Saul Carcamo, Valentina Kirigin Callaú, Shannon Moran, Dan Filipescu, Somnath Tagore, Tessa M. Firestone, Michael-Christopher Keogh, Lu Sun, Benjamin Izar, Dan Hasson, Emily Bernstein
Molecular Cell Published: April 18, 2025
DOI:https://doi.org/10.1016/j.molcel.2025.03.026
Graphical abstract

Highlights
- PBAF regions are less sensitive to ATPase-dependent remodeling than those of BAF
- A subset of PBAF regions colocalize with Polycomb repressive complexes and REST
- The TF REST relies on PBAF to bind chromatin and silence its target neuronal genes
- ARID2 mutations correlate with a REST synaptic gene signature in melanoma patients
Summary
SWI/SNF (switch/sucrose non-fermentable) chromatin remodelers possess unique functionalities difficult to dissect. Distinct cancers harbor mutations in specific subunits, such as the polybromo-associated BAF (PBAF)-specific component ARID2 in melanoma. Here, we perform epigenomic profiling of SWI/SNF complexes and their associated chromatin states in melanocytes and melanoma. Time-resolved approaches reveal that PBAF regions are generally less sensitive to ATPase inhibition than BAF sites. We further uncover a subset of PBAF-exclusive regions within Polycomb-repressed chromatin that are enriched for REST (RE1 silencing transcription factor), a transcription factor that represses neuronal genes. In turn, PBAF complex disruption via ARID2 loss hinders REST’s ability to bind and inactivate its targets, leading to upregulation of synaptic transcripts. Remarkably, this gene signature is conserved in melanoma patients with ARID2 mutations and correlates with an expression program enriched in melanoma brain metastases. Overall, we demonstrate a unique role for PBAF in generating accessibility for a silencing transcription factor at repressed chromatin, with important implications for disease.