2026-02-11 ペンシルベニア州立大学(Penn State)
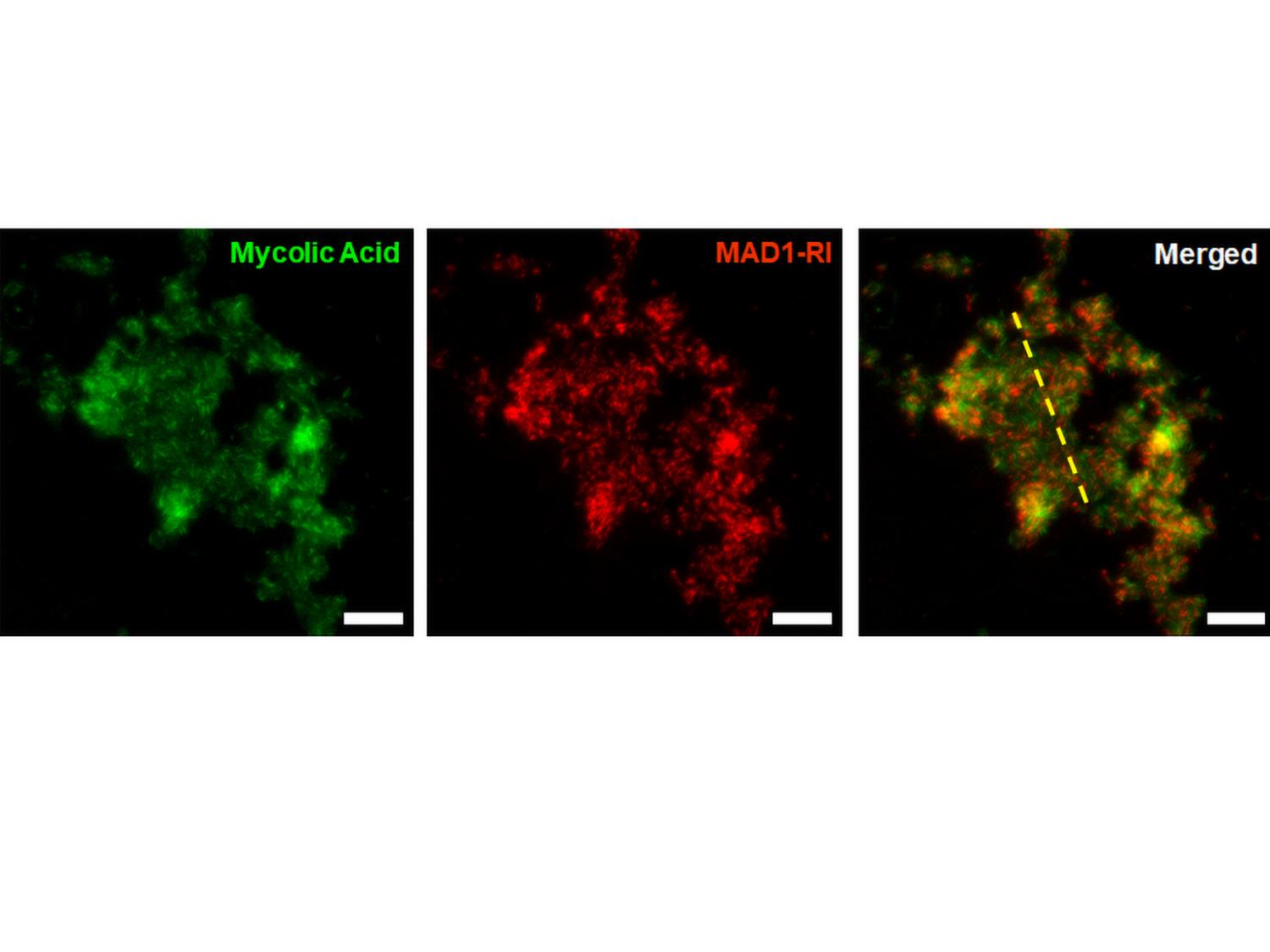
A triptych of microscope images that shows the binding of a host defense peptide, named MAD1-RI, to the membrane of tuberculosis pathogens, leading to destruction of the bacterial cell wall and death. Credit: Scott Medina / Penn State. Creative Commons
<関連情報>
- https://www.psu.edu/news/huck-institutes-life-sciences/story/flipping-and-reversing-mini-proteins-could-improve-disease
- https://www.nature.com/articles/s41467-025-67162-0
Retro-inversion imparts antimycobacterial specificity to host defense peptides
Hugh D. Glossop,Gebremichal Gebretsadik,Sabiha Sultana,Diptomit Biswas,Nathan A. Schacht,Neela H. Yennawar,Muzafar Ahmad Rather,Anthony D. Baughn & Scott H. Medina
Nature Communications Published:07 December 2025
DOI:https://doi.org/10.1038/s41467-025-67162-0
Abstract
Antimicrobial host defense peptides are promising alternatives to resistance prone small molecule antibiotics. To overcome the poor physiologic stability of these therapeutic candidates it is common to prepare proteolytically resistant retro-inverso analogues, where sequence backbone direction and amino acid chirality are reversed. However, in many cases, gains in stability are offset by altered assembly propensities and reduced biologic potency. Here, we show that, contrary to the dogma for non-mycobacterial pathogens, retro-inversion of antimycobacterial host defense peptides improves their potency, specificity and host safety; in some cases by more than an order of magnitude. Biophysical assays suggest that altered mycomembrane thermodynamics, instead of improved proteolytic stability, plays a causative role in retro-inverso mediated potency gains. Additional bacteriologic assays using a lead retro-inversed candidate, MAD1-RI, demonstrate this analogue rapidly sterilizes replicating cultures of Mycobacterium tuberculosis, is effective towards drug-resistant clinical isolates of the pathogen, and synergistically enhances the activity of co-incubated antibiotics. Transcriptomic studies uncover complementary membrane destabilizing and metabolic mechanisms of antitubercular action for MAD1-RI, and in doing so identify sequence retro-inversion as a simple, but powerful, modality in the de novo design of non-natural antimycobacterial peptides.