2023-08-30 カリフォルニア大学サンディエゴ校(UCSD)
◆研究によると、これらの細胞は食事に敏感であり、膨大なコレステロール生成装置を持つ一方、コエンザイムQというエネルギー増強分子も生成し、細胞のバッテリーであるミトコンドリアを活性化する役割を果たすことが分かりました。これに基づき、既存の薬剤を活用してこれらの細胞の代謝を調整し、免疫細胞の能力を向上させる方法が提案されています。
<関連情報>
- https://today.ucsd.edu/story/enhancing-immune-defenses-researchers-unveil-the-secrets-of-specialized-t-cells-to-conquer-tumors
- https://www.nature.com/articles/s41586-023-06483-w
T細胞組織滞留の代謝プログラムが腫瘍免疫に力を与える Metabolic programs of T cell tissue residency empower tumour immunity
Miguel Reina-Campos,Maximilian Heeg,Kelly Kennewick,Ian T. Mathews,Giovanni Galletti,Vida Luna,Quynhanh Nguyen,Hongling Huang,J. Justin Milner,Kenneth H. Hu,Amy Vichaidit,Natalie Santillano,Brigid S. Boland,John T. Chang,Mohit Jain,Sonia Sharma,Matthew F. Krummel,Hongbo Chi,Steven J. Bensinger & Ananda W. Goldrath
Nature Published:30 August 2023
DOI:https://doi.org/10.1038/s41586-023-06483-w
Abstract
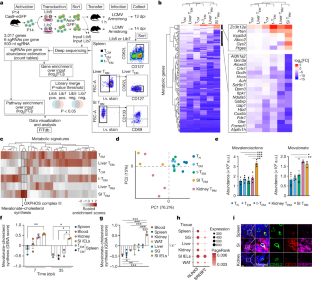
Tissue resident memory CD8+ T (TRM) cells offer rapid and long-term protection at sites of reinfection1. Tumour-infiltrating lymphocytes with characteristics of TRM cells maintain enhanced effector functions, predict responses to immunotherapy and accompany better prognoses2,3. Thus, an improved understanding of the metabolic strategies that enable tissue residency by T cells could inform new approaches to empower immune responses in tissues and solid tumours. Here, to systematically define the basis for the metabolic reprogramming supporting TRM cell differentiation, survival and function, we leveraged in vivo functional genomics, untargeted metabolomics and transcriptomics of virus-specific memory CD8+ T cell populations. We found that memory CD8+ T cells deployed a range of adaptations to tissue residency, including reliance on non-steroidal products of the mevalonate–cholesterol pathway, such as coenzyme Q, driven by increased activity of the transcription factor SREBP2. This metabolic adaptation was most pronounced in the small intestine, where TRM cells interface with dietary cholesterol and maintain a heightened state of activation4, and was shared by functional tumour-infiltrating lymphocytes in diverse tumour types in mice and humans. Enforcing synthesis of coenzyme Q through deletion of Fdft1 or overexpression of PDSS2 promoted mitochondrial respiration, memory T cell formation following viral infection and enhanced antitumour immunity. In sum, through a systematic exploration of TRM cell metabolism, we reveal how these programs can be leveraged to fuel memory CD8+ T cell formation in the context of acute infections and enhance antitumour immunity.